Abstract
Inherited cardiac conditions (ICCs) are characterised by marked genetic and allelic heterogeneity and require extensive sequencing for genetic characterisation. We iteratively optimised a targeted gene capture panel for ICCs that includes disease-causing, putatively pathogenic, research and phenocopy genes (n = 174 genes). We achieved high coverage of the target region on both MiSeq (>99.8 % at ≥20× read depth, n = 12) and NextSeq (>99.9 % at ≥20×, n = 48) platforms with 100 % sensitivity and precision for single nucleotide variants and indels across the protein-coding target on the MiSeq. In the final assay, 40 out of 43 established ICC genes informative in clinical practice achieved complete coverage (100 % at ≥20×). By comparison, whole exome sequencing (WES; ∼80×), deep WES (∼500×) and whole genome sequencing (WGS; ∼70×) had poorer performance (88.1, 99.2 and 99.3 % respectively at ≥20×) across the ICC target. The assay described here delivers highly accurate and affordable sequencing of ICC genes, complemented by accessible cloud-based computation and informatics. See Editorial in this issue (DOI: 10.1007/s12265-015-9667-8).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited cardiac conditions (ICCs) are diseases of the heart and circulation with a combined prevalence of ∼1 %. ICCs include inherited arrhythmia syndromes, cardiomyopathies, aortopathies and hyperlipidaemias [1–4]. They most commonly exhibit autosomal dominant inheritance, though with highly variable expressivity and penetrance. Sequencing of ICC genes can be performed to confirm an ICC diagnosis, inform patient management/cascade screening and be useful for molecular autopsy in the case of sudden unexplained death [5].
Until recently, Sanger sequencing was used for ICC gene sequencing for both clinical and research applications, but this technique has limited throughput and is prohibitively expensive for large genes/large numbers of genes [6]. Next-generation sequencing (NGS) reduces the cost and increases throughput of gene sequencing and can now be performed on inexpensive bench-top NGS platforms [7]. Bench-top sequencers have the advantages of low capital cost, compact footprint and a simplified workflow compared to high-throughput sequencers, yet still meet the sequencing needs of individual research and clinical laboratories [8].
Whole exome sequencing (WES) and targeted sequencing have been developed as alternatives to whole genome sequencing (WGS). These approaches have reduced sequencing costs, turnaround times, data storage needs and informatics burdens compared to WGS. There are many approaches to enrich for target sequences that use varying DNA preparation and capture methods that can be in solution, solid-phase or PCR-based [9]. In solution, WES is a popular off-the-shelf choice, as assays have been designed to capture all human genes. However, WES often results in uneven coverage across and between genes and can particularly struggle with GC-rich regions such as first exons [10]. The interpretation of incidental variants, as suggested by American College of Medical Genetics and Genomics (ACMG), is also a potential issue for WES where variants unrelated to the patient’s referral condition may be detected [11]. Augmented WES assays containing additional probes targeting disease genes have been developed, but average assay performance remains suboptimal (∼90 %, ≥20×) and WGS may perform better [12].
Targeted sequencing of gene panels is an alternative to WES and has been widely used in research and is increasingly applied in clinical settings [13]. In the ICC setting, small gene panels have been used for specific ICCs, including long QT syndrome (LQTS), hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) [14–16]. Multiple workflows and bioinformatics pipelines are needed to run these various ICC gene panels, and gene coverage is such that Sanger sequencing ‘fill in’ is needed, which has very major manpower implications.
Here, we describe the development of a new gene panel for ICCs, which provides a comprehensive, single workflow assay with high levels of coverage across all ICC genes for use in research and ultimately clinical settings. The performance of the panel was iteratively improved by probe design, across sequencing platforms and by sequencing chemistry refinement. Assay performance was assessed in detail compared to WES and WGS using local and cloud-based informatics pipelines.
Materials and Methods
Subject Specimens
Subjects (n = 348) were recruited from National Heart Centre Singapore and via advertisement at the MRC Clinical Sciences Centre, Imperial College London. Samples for WGS (n = 8) were obtained from National Cancer Centre Singapore, National University Hospital Singapore. All participants gave written informed consent, and study protocols were approved by the local institutional ethics committees and carried out in accordance with local Tissue Acts, as appropriate. Genomic DNA was extracted from blood using Prepito DNA Blood 600 kit (Perkin Elmer, MA) (targeted sequencing), EZ1 DSP DNA blood 48 kit (Qiagen, Netherlands) (WES) or QIAsymphony DNA kit (Qiagen, Netherlands) (WGS) following manufacturer’s protocols. Quality and quantity of extracted DNA were assessed by an ultraviolet-visible spectrophotometer.
Targeted Enrichment
An initial ICC gene panel targeting 169 ICC genes (ICCv1, target region = 1.47 Mb; including 3′ and 5′UTRs) and an iterated version targeting 174 genes (ICCv2, target region = 0.57 Mb; protein coding ± 40 bp buffer) were designed using Illumina Design Studio (San Diego, CA). Genes were chosen on the basis of reported associations of disease-causing variants with relevant ICCs which were identified in the Human Gene Mutation Database (HGMD) Professional version 2014.1, followed by manual curation and addition of further genes of research interest by a team of cardiologists and clinical geneticists (Table S1). ICCv2 BED file with targets and genomic coordinates are provided in Table S2. The 169 ICC genes consistently represented in all sequence capture panels were assessed for the purposes of this study. Libraries were prepared using Nextera Rapid Capture Enrichment kits according to the manufacturer’s protocols.
Targeted, Whole Exome and Whole Genome Sequencing
Targeted sequencing: Pooled libraries (n samples = 6–48) prepared using the ICC panel were sequenced on the Illumina MiSeq (v2 kit; n = 108) or NextSeq 500 (Mid Output v2 kit, n = 144) benchtop sequencers using paired-end, 150 bp reads. WES: 96 samples underwent WES using the Nextera Rapid Capture Exome kit according to the manufacturer’s instructions. Each pool (n = 12) was sequenced on a single lane of the HiSeq 2500 (SBS v4 kit, 125 bp paired-end (PE) reads, yielding ≥4 GB of raw data per sample, ∼mean read depth of 80× and >80 % of bases at >10×). Deep WES: Six out of 96 WES samples were randomly selected, and all reads were combined to obtain sequencing depth equivalent to that acquired by ICC panel sequencing in typical use (∼43 GB of raw data per sample, ∼mean read depth of 500×). WGS: Eight samples were prepared using the TruSeq Nano DNA kit according to the manufacturer’s instructions. Each sample was sequenced on two lanes of the HiSeq X (v2.5 kit, 150 bp PE reads, yielding ∼200 GB of raw data per sample, ∼mean read depth of 70×).
Sequence Alignment and Variant Calling
Raw sequencing data (.bcl files) were demultiplexed into individual FastQ read files with Illumina’s bcl2fastq v2.16.0.10 based on unique index pairs. Low quality (Q < 20) reads/bases were trimmed using Trimmomatic v0.3220.4 [17], and read quality was assessed using FastQC v0.10.1 [18]. High-quality reads were mapped to UCSC GRCh37/hg19 reference genome using Burrows-Wheeler Aligner (BWA) v0.7.10 [19]. Picard v1.119 and The Genome Analysis Toolkit (GATK) v3.3 [20] were used to mark duplicate reads, realign locally around indels and recalibrate base quality scores according to best practices. Alignment summary metrics and coverage and callability metrics were generated using Picard v1.119, SAMtools v1.1 [21], Bedtools v2.17 [22] and in-house Perl/Shell scripts. A base was considered ‘callable’ if sequenced with minimum read depth = 20×, base quality ≥ 20 and mapping quality ≥ 20. GATKv3.3 HaplotypeCaller and UnifiedGenotyper were used to call variants from reads mapped with quality ≥ 20. Variants were annotated with Ensembl Variation database v75_37 [23] and HGMD Professional version 2014.1 [24]. Among all 252 samples sequenced using the ICC panel, there were ten outliers (defined as total number of reads per sample greater than third quartile +1.5 inter-quartile range (IQR) or below first quartile −1.5 IQR), which were excluded from the analysis. In addition, 11 WES samples with <80 % of bases at >10× were excluded from analysis. Pathogenic or likely pathogenic variants (n = 26) identified using the ICC panel in a research cohort (n = 35) were subjected to Sanger sequencing. In addition to our in-house pipeline described above, a subset of samples (n = 65) were also analysed using the BWA Enrichment v2.1 and Isaac Enrichment v2.1 available in Illumina’s cloud genomics platform (https://basespace.illumina.com), and variant calling data was compared to the in-house GATK HaplotypeCaller pipeline (Table S3) [25, 26].
Sensitivity and Precision of Variant Calling
Sensitivity and precision of variant calling of the ICC panel were assessed using the NA12878 reference sample. High confidence regions and the associated variant calls were downloaded from Genome in a Bottle (GIAB) (ftp://ftp-trace.ncbi.nlm.nih.gov/giab/ftp/release/NA12878_HG001/NISTv2.19/) [27] and compared to variant calls from ICC panel sequencing on both the MiSeq and NextSeq platforms. Variant calls were defined as true positive (TP) for those identified from panel sequencing and by GIAB, false positive (FP) for those identified as reference by GIAB but as variant in panel sequencing, false negative (FN) for variants identified by GIAB but not by panel sequencing and true negative (TN) for bases identified as reference in both the GIAB call set and panel sequencing. Sensitivity was calculated as TP / (TP + FN) and precision as TP / (TP + FP). Finally, we calculated the Matthews correlation coefficient (MCC), an alternative accuracy measure that takes into account unbalanced data, using the following equation: (TP × TN) − (FP × FN) / √[(TP + FP)(TP + FN)(TN + FP)(TN + FN)].
Results
ICC Panel: Optimisation and Performance
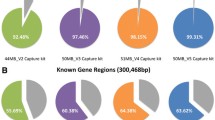
The performance across the iteratively improved ICC gene panels was compared using a callability metric (minimum read depth = 20×, base quality ≥ 20 and mapping quality ≥ 20) that defines adequate coverage for robust variant calling. Four methods were compared (Table S4). First, ICCv1 (169 genes, 1.47 Mb target) was sequenced at standard multiplex (method 1; M1). The mean callability of all genes using ICCv1 was proportional to the number of mapped reads per sample at low depth but saturated at ∼4 M mapped reads per sample. The low overall performance of M1 (∼94 % target, mean read depth > 20×) reflected low capture efficiency of specific gene regions as opposed to a global effect. In an attempt to improve assay performance, we included fewer samples per run (n = 6, method 2 (M2)) resulting in better performance (99.8 % target, ≥20×) but at a greater cost (Table S4). Overall, the performance of the M1 and M2 assays were suboptimal and are not referred to further.
We then made a major iteration of the target capture assay in ICCv2 by reducing the target (size = 0.57 Mb) to the coding DNA sequence only and by modifying the baits targeting poorly captured regions. The ICCv2 assay consisted of 174 genes, of which 169 genes were shared with the ICCv1 panel (169 genes, size = 0.56 Mb) and are considered in the comparisons presented here (Table S1). Libraries prepared with ICCv2 were sequenced either on the MiSeq (method 3, (M3)) or the NextSeq 500 (method 4, (M4)). Both M3 and M4 achieved major improvements in overall performance when compared to M1 and M2, in additional to a reduced sequencing cost per sample (Table S4).
MiSeq Versus NextSeq 500 Sequencing
The maximal data output of the MiSeq was 6.6 GB (up to 42.1 million paired-end reads passing filters), while the NextSeq 500 generated up to 65 GB of data (up to 420 million paired-end reads passing filters). Limited duplicate reads (∼15 %) were observed with MiSeq runs, while a twofold increased duplicate reads were found (∼30 %) with NextSeq 500 runs, likely reflecting limited library complexity (fragment start sites and insert sizes of PE reads). Using ICCv2 and the NextSeq platform, the coverage of well-characterised, disease-causing genes across the major ICC disease classes was 100 % for 40 out of the 43 genes (Table 1). Small recurrent gaps in gene coverage occurred in three important genes: TGFBR1 (97 bp, exon 1), MYH7 (160 bp, exon 27) and TTN (72 to 90 bp, 19 exons (168–252)) (Table 1).
Comparison Between Targeted ICC Sequencing, WES, Deep WES and WGS
We then compared the performance of the final assay (ICCv2) against Nextera-based WES and WGS (Table 2; Fig. 1). Using routinely applied, off-the-shelf WES, most disease-causing ICC genes (n = 36/43) had suboptimal coverage (49–98 %, 20×). For a direct comparison at the same level of read depth as the ICC methods, deep WES (∼500×) was used. However, even with deep WES, ten disease genes remained poorly covered, an average of 208 bases had no coverage at all and the cost was extremely high ($5400). The performance of WGS at ∼75× average read depth was similar to deep WES at ∼520× read depth but at lower cost.
Stringent heat map showing the percentage coverage of ICC disease genes commonly used to inform clinical practice (n = 43) at 20× read depth using M3 (MiSeq, 150 bp PE), M4 (NextSeq 500, 150 bp PE), deep whole exome sequencing (WES; HiSeq 2500, 125 bp PE), WES (HiSeq, 125 bp PE) and whole genome sequencing (WGS: HiSeq X, 150 bp PE) (gene coverage at 20×: dark red ≤98 %; dark green = 100 %)
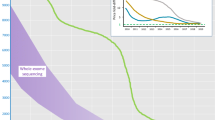
By comparison, using the ICCv2 assay (M4, NextSeq), only three of the major ICC disease genes were not 100 % covered at 20× and an average of only 22 bases of target were not covered at all (Fig. 1, Table 1). Gene complexity was a major determinant of base coverage, especially for regions of high GC content and low mappability in the titin gene (Fig. 2) [29]. Perhaps surprisingly, mean read depth coverage of titin exons using deep WES was higher than M4 for regions of low complexity, perhaps reflecting greater library complexity for deep WES. However, the overall titin gene coverage at a mean read depth of 20× was best with our final assay on the NextSeq (99.7 %), less good with deep WES (99.0 %) and WGS (99.2 %) and poor with standard WES (85.1 %).
Percentage coverage of all TTN exons (ENST00000589042/NM_001267550.1) at 20× read depth across methods (top four panels). Mappability score (score* [28]) and GC content in the TTN gene (bottom two panels). Error bars represent standard deviation
Variant Calling Accuracy
Variant calling accuracy was assessed using an in-house pipeline using the reference NA12878 sample. Variants were assessed over a 522,763 bp region overlapping with the GIAB high confidence regions and which corresponded to the ICC target ±8 bps (to include essential splice site and proximal intronic regions). The ICC panel had very high accuracy as compared to GIAB benchmark calls dataset (Table 3). The MiSeq and NextSeq assays had near identical performance with SNV sensitivity of 100 %. The NextSeq data had one false positive that only just passed the minimum variant confidence/quality by depth (QD) threshold of 2.0 (QD = 2.1) and was of obvious low quality when viewed in IGV. The false negative indel missed by both platforms was an A deletion, 6 bp into an intron and before a run of 15 As. This variant was initially called on the MiSeq platform but filtered out due to low QD (chr12 22063251 CA C).
Variant Calling Comparisons
Single nucleotide variant (SNV) calling was assessed using the DNA substitution rate, i.e. the ratio of transitions (Ts) to transversions (Tv). We observed a Ts/Tv ratio ∼3.5 across the targeted CDS region for our assays, concordant with previous findings [30]. A total of 65 samples from M3 (MiSeq, n = 23) and M4 (NextSeq 500, n = 42) were selected for variant calling comparisons using either our in-house pipeline (The GATK Best Practices workflow) or one of two Illumina BaseSpace Apps: Isaac enrichment v2.1 or BWA enrichment v2.1 (Table S5) [31]. For 65 samples, BaseSpace Apps completed jobs within 1 h as compared to locally run pipelines that took ∼2 h on a computational cluster (local cluster four CPU cores per job with 14 GB/CPU RAM allocation). We observed 98.8 % concordance between our in-house pipeline and both BaseSpace Apps for SNVs and indels with ∼100 % of SNVs detected locally also detected by both apps. A subset of 26 variants identified by our custom pipeline underwent Sanger sequencing and were all confirmed.
Variant calling assessment test (VCAT) [BaseSpace App, Illumina Inc.] was performed on variant call sets obtained from all three pipelines using reference sample NA12878 (n = 16, technical replicates). After comparing with gold standard GIAB high confidence calls v2.18 and Platinum genome v8.0, we observed 100 % precision for SNV and indel calls obtained from both the in-house, custom pipeline and BWA Enrichment app in BaseSpace. However, Isaac Enrichment variant call set had poorer performance with 97 % and below 50 % precision for SNV and indel calls respectively (Table S6).
Discussion
In recent years, the use of targeted sequencing and WES for the study of ICCs has increased, reflecting high-throughput capabilities and reduced per-base costs of NGS when compared to conventional Sanger sequencing. Current NGS cardiac panels often represent a limited number of ICC genes (n = 9 to 88), and assay performance is variable, often requiring PCR-based gap filling and Sanger sequencing [15, 32–34]. The final ICC assay presented here includes 174 ICC genes that have primary, secondary or possible involvement in a wide range (>17) of ICCs (Table S1) including all 30 ACMG genes [11] and phenocopy genes [35]. However, common pathogenic variants that are outside the captured region cannot be assessed by this assay, for instance, the 25 bp deletion in intron 32 of MYBPC3 that has been associated with HCM [36].
The assay we describe represents over 85 genes implicated in cardiomyopathies including all major disease and phenocopy genes for HCM, DCM and ARVC [2, 37, 38]. Inherited arrhythmias are an important group of ICCs, and over 28 genes implicated in inherited arrhythmias including all major disease-causing genes for LQTS are included in the assay (Table 1, Table S1). The panel also includes over 14 genes implicated in inherited aortopathies, 12 of which are common to an established panel for thoracic aortic aneurysm [39]. The comprehensive nature of the panel we describe here makes it ideally suited for a single workflow in laboratories providing sequencing for multiple ICCs and for molecular pathology studies of sudden cardiac death [40, 41], although more specialised panels may offer advantages in focused/single disease laboratories. It is interesting to note that from a clinical point of view, the major disease-causing ICC genes used to inform clinical practise have not changed much over recent years. While the current panel is of fixed content, it would be possible to iterate the design in the future and to include intronic regions of interest, if the ICC community was to solicit this change.
It could, and has been, argued that simply using off-the-shelf WES is sufficient for ICC research and diagnostics [42]. However, the coverage of ICC genes using WES at manufacturer-recommended sequencing depths is insufficient for accurate variant calling for a number of ICC genes (Table 2, Fig. 1). Even with deep WES (∼500× read depth across ICC target), ICC gene coverage was less good than the optimised ICC-specific assay (Fig. 1). WGS may be better than WES for detecting exome variants [43], and we found the coverage of ICC genes to be good, but WGS comes with cost, incidental finding and data storage issues [44], and at an average of 70× coverage is not as good for ICC gene assessment as the assay we describe here.
It is important to consider differences in variant calling between informatic pipelines as highlighted by our comparison of three methods that use different mapping and variant calling algorithms and data pre-processing workflows. It has been reported that alignment with BWA-MEM and GATK HaplotypeCaller pipeline offers best sensitivity and precision [45]. Cloud-based and easily implemented pipelines on BaseSpace offer a viable alternative for those with limited in-house informatics and, based on preliminary analyses, have comparable sensitivity (Table S5). We suggest that individual users prioritise and use one pipeline and then work to identify pipeline-specific performance parameters. An advantage of using cloud-based processes is that computational hardware purchase and upkeep is not needed and the processing power accessible via BaseSpace is fast. Hence, small laboratories can readily access both processing power and informatics tools.
In summary, the ICC gene panel described here provides high and uniform coverage (99.9 % targeted region at >20×), ‘clinical-grade’ sequencing with up to 100 % sensitivity and precision for SNVs and indels in the protein-coding regions of ICC genes. This raises the question as to whether or not ‘Sanger validation’ is required as part of a clinical workflow; the data presented here would suggest not. As compared to the WES, deep WES and WGS, this assay has better performance, shorter turnaround times, lesser informatics requirements and lower sequencing costs. While assessment of structural variation remains a challenge, the very deep coverage this panel affords may provide ways to interrogate this in the future. We believe that this panel will be important for ICC research and ultimately clinical genetic investigation of ICCs and for molecular autopsy. This panel is now available commercially (TruSight Cardio Sequencing Kit; research use only), and with the ease of use of cloud-based computational processing and informatics, it is widely accessible for users.
References
Wang, Q., Curran, M. E., Splawski, I., et al. (1996). Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nature Genetics, 12(1), 17–23. doi:10.1038/ng0196-17.
Herman, D. S., Lam, L., Taylor, M. R. G., et al. (2012). Truncations of titin causing dilated cardiomyopathy. New England Journal of Medicine, 366(7), 619–628. doi:10.1056/NEJMoa1110186.
Dietz, H. C., Cutting, C. R., Pyeritz, R. E., et al. (1991). Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature, 352(6333), 337–339. doi:10.1038/352337a0.
Van Wingerden, J. J. (1981). Primary familial hypercholesterolaemia in a South African black. A case report. South African Medical Journal = Suid-Afrikaanse Tydskrif vir Geneeskunde, 60(14), 554–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7280910 .
Antzelevitch, C., Pollevick, G. D., Cordeiro, J. M., et al. (2007). Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation, 115, 442–449. doi:10.1161/CIRCULATIONAHA.106.668392.
Kingsmore, S. F., & Saunders, C. J. (2011). Deep sequencing of patient genomes for disease diagnosis: when will it become routine? Science Translational Medicine, 3, 87ps23. doi:10.1126/scitranslmed.3002695.
Ware, J. S., Roberts, A. M., & Cook, S. A. (2012). Next generation sequencing for clinical diagnostics and personalised medicine: implications for the next generation cardiologist. Heart, 98(4), 276–281. doi:10.1136/heartjnl-2011-300742.
Li, X., Buckton, A. J., Wilkinson, S. L., et al. (2013). Towards clinical molecular diagnosis of inherited cardiac conditions: a comparison of bench-top genome DNA sequencers. PLoS One, 8(7), e67744. doi:10.1371/journal.pone.0067744.
Chilamakuri, C. S. R., Lorenz, S., Madoui, M., et al. (2014). Performance comparison of four exome capture systems for deep sequencing. BMC Genomics. doi:10.1186/1471-2164-15-449.
Lan, J. H., Yin, Y., Reed, E. F., et al. (2015). Impact of three Illumina library construction methods on GC bias and HLA genotype calling. Human Immunology, 76(2–3), 166–75. doi:10.1016/j.humimm.2014.12.016.
Green, R. C., Berg, J. S., Grody, W. W., et al. (2013). ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 15(7), 565–74. doi:10.1038/gim.2013.73.
Patwardhan, A., Harris, J., Leng, N., et al. (2015). Achieving high-sensitivity for clinical applications using augmented exome sequencing. Genome Medicine, 7(1), 71. doi:10.1186/s13073-015-0197-4.
Voelkerding, K. V., Dames, S., & Durtschi, J. D. (2010). Next generation sequencing for clinical diagnostics-principles and application to targeted resequencing for hypertrophic cardiomyopathy: a paper from the 2009 William Beaumont Hospital Symposium on Molecular Pathology. The Journal of Molecular Diagnostics: JMD, 12(5), 539–551. doi:10.2353/jmoldx.2010.100043.
Millat, G., Chanavat, V., & Rousson, R. (2014). Evaluation of a new high-throughput next-generation sequencing method based on a custom AmpliSeqTM Library and Ion Torrent PGMTM sequencing for the rapid detection of genetic variations in long QT syndrome. Molecular Diagnosis & Therapy. doi:10.1007/s40291-014-0099-y.
Akinrinade, O., Ollila, L., Vattulainen, S., et al. (2015). Genetics and genotype-phenotype correlations in Finnish patients with dilated cardiomyopathy. European Heart Journal, 2327–2337. doi:10.1093/eurheartj/ehv253.
Gréen, A., Gréen, H., Rehnberg, M., et al. (2014). Assessment of HaloPlex amplification for sequence capture and massively parallel sequencing of arrhythmogenic right ventricular cardiomyopathy-associated genes. The Journal of Molecular Diagnostics: JMD, 17(1), 1–13. doi:10.1016/j.jmoldx.2014.09.006.
Schmieder, R., & Edwards, R. (2011). Quality control and preprocessing of metagenomic datasets. Bioinformatics, 27, 863–864. doi:10.1093/bioinformatics/btr026.
Andrews, S. (2010). FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, 1. Retrieved from http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:FastQC+a+quality+control+tool+for+high+throughput+sequence+data.#0.
Li, H., & Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25, 1754–1760. doi:10.1093/bioinformatics/btp324.
McKenna, A., Hanna, M., Banks, E., et al. (2010). The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research, 20, 1297–1303. doi:10.1101/gr.107524.110.
Li, H., Handsaker, B., Wysoker, A., et al. (2009). The sequence alignment / map format and SAMtools. Bioinformatics, 25, 2078–2079. doi:10.1093/bioinformatics/btp352.
Quinlan, A. R., & Hall, I. M. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics, 26, 841–842. doi:10.1093/bioinformatics/btq033.
McLaren, W., Pritchard, B., Rios, D., et al. (2010). Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics, 26, 2069–2070. doi:10.1093/bioinformatics/btq330.
Stenson, P. D., Ball, E. V., Mort, M., et al. (2003). Human Gene Mutation Database (HGMD®): 2003 update. Human Mutation. doi:10.1002/humu.10212.
Li, H. (2011). Tabix: fast retrieval of sequence features from generic TAB-delimited files. Bioinformatics, 27(5), 718–719. doi:10.1093/bioinformatics/btq671.
Danecek, P., Auton, A., Abecasis, G., et al. (2011). The variant call format and VCFtools. Bioinformatics, 27(15), 2156–2158. doi:10.1093/bioinformatics/btr330.
Zook, J. M., Chapman, B., Wang, J., et al. (2014). Integrating human sequence data sets provides a resource of benchmark SNP and indel genotype calls. Nature Biotechnology, 32(3), 246–51. doi:10.1038/nbt.2835.
Derrien, T., Estellé, J., Marco Sola, S., et al. (2012). Fast computation and applications of genome mappability. PLoS One, 7(1), e30377. doi:10.1371/journal.pone.0030377.
Ware, J. S., John, S., Roberts, A. M., et al. (2013). Next generation diagnostics in inherited arrhythmia syndromes: a comparison of two approaches. Journal of Cardiovascular Translational Research, 6, 94–103. doi:10.1007/s12265-012-9401-8.
Marth, G. T., Yu, F., Indap, A. R., et al. (2011). The functional spectrum of low-frequency coding variation. Genome Biology, 12(9), R84. doi:10.1186/gb-2011-12-9-r84.
Raczy, C., Petrovski, R., Saunders, C. T., et al. (2013). Isaac: ultra-fast whole-genome secondary analysis on Illumina sequencing platforms. Bioinformatics, 29(16), 2041–2043. doi:10.1093/bioinformatics/btt314.
Millat, G., Chanavat, V., & Rousson, R. (2014). Evaluation of a new NGS method based on a custom AmpliSeq library and Ion Torrent PGM sequencing for the fast detection of genetic variations in cardiomyopathies. Clinica Chimica Acta; International Journal of Clinical Chemistry, 433, 266–71. doi:10.1016/j.cca.2014.03.032.
Glotov, A. S., Kazakov, S. V., Zhukova, E. A., et al. (2015). Targeted next-generation sequencing (NGS) of nine candidate genes with custom AmpliSeq in patients and a cardiomyopathy risk group. Clinica Chimica Acta, 446, 132–140. doi:10.1016/j.cca.2015.04.014.
Wilson, K. D., Shen, P., Fung, E., et al. (2015). A rapid, high-quality, cost-effective, comprehensive and expandable targeted next-generation sequencing assay for inherited heart diseasesnovelty and significance. Circulation Research, 117(7), 603–611. doi:10.1161/CIRCRESAHA.115.306723.
Rehm, H. L. (2013). Disease-targeted sequencing: a cornerstone in the clinic. Nature Reviews Genetics, 14(4), 295–300. doi:10.1038/nrg3463.
Kuster, D. W. D., Govindan, S., Springer, T. I., et al. (2015). A hypertrophic cardiomyopathy-associated\n MYBPC3\n mutation common in populations of South Asian descent causes contractile dysfunction. Journal of Biological Chemistry, 290(9), 5855–5867. doi:10.1074/jbc.M114.607911.
Alfares, A. A., Kelly, M. A., McDermott, G., et al. (2015). Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genetics in Medicine. doi:10.1038/gim.2014.205.
Roberts, A. M., Ware, J. S., Herman, D. S., et al. (2015). Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease, 7(270), 1–15.
Proost, D., Vandeweyer, G., Meester, J. A. N., et al. (2015). Performant mutation identification using targeted next-generation sequencing of 14 thoracic aortic aneurysm genes. Human Mutation, 36(8), 808–814. doi:10.1002/humu.22802.
Juan Fernando Agudelo, G. M. P. (2014). Study of a Colombian Family with Hypertrophic Cardiomyopathy and Sudden Cardiac Death Associated with the Lys247arg Mutation in the Cardiac Troponin T (Tnnt2) Gene: Casual Relationship or Polymorphism? Journal of Molecular and Genetic Medicine, 08(02). doi:10.4172/1747-0862.1000112
Pan, H., Richards, A. A., Zhu, X., et al. (2009). A novel mutation in LAMIN A/C is associated with isolated early-onset atrial fibrillation and progressive atrioventricular block followed by cardiomyopathy and sudden cardiac death. Heart Rhythm, 6(5), 707–710. doi:10.1016/j.hrthm.2009.01.037.
Chugh, S. S., & Huertas-Vazquez, A. (2014). Inherited arrhythmia syndromes: exome sequencing opens a new door to diagnosis. Journal of the American College of Cardiology, 63(3), 267–268. doi:10.1016/j.jacc.2013.07.089.
Belkadi, A., Bolze, A., Itan, Y., et al. (2015). Whole-genome sequencing is more powerful than whole-exome sequencing for detecting exome variants. Proceedings of the National Academy of Sciences of the United States of America, 1418631112. doi:10.1073/pnas.1418631112.
Dewey, F. E., Grove, M. E., Pan, C., et al. (2014). Clinical interpretation and implications of whole-genome sequencing. JAMA : The Journal of the American Medical Association, 311(10), 1035–45. doi:10.1001/jama.2014.1717.
Highnam, G., Wang, J. J., Kusler, D., et al. (2015). An analytical framework for optimizing variant discovery from personal genomes. Nature Communications, 6, 6275. doi:10.1038/ncomms7275.
Acknowledgments
This publication includes independent research commissioned by the Health Innovation Challenge Fund (HICF), a parallel funding partnership between the Department of Health and Wellcome Trust. The views expressed in this work are those of the authors and not necessarily those of the Department of Health or Wellcome Trust. We thank Dr. Tony Lim from Department of Pathology, Singapore General Hospital for access to NextSeq sequencing, all participants recruited from NHCS and MRC Clinical Sciences Centre, Imperial College London for involving in this research and our team of clinical research coordinators on both hospital sites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of Funding
This study was supported by The National Medical Research Council of Singapore, Goh foundation, Tanoto Foundation, SingHealth and Duke-NUS Precision Medicine Institute, The UK Medical Research Council, NIHR Cardiovascular BRU at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, British Heart Foundation Wellcome Trust and Health Innovation Challenge Fund (HICF-R6-373) funding from the Wellcome Trust and Department of Health, UK.
Conflict of Interest
The panel was developed in collaboration with Illumina in its later iterations with respect to bait design, and Illumina personnel performed one targeted sequencing on reference DNA (NA12878) using Trusight Cardio (ICCv2) panel and NextSeq 500. Prof. Cook has previously consulted for Illumina.
Human Subjects/Informed Consent Statement
All participants gave written informed consent, and study protocols were approved by the local institutional ethics committees or in accordance with local Tissue Acts, as appropriate.
Animal Studies
No animal studies were carried out by the authors for this article.
Additional information
Editor-in-Chief Jennifer L. Hall oversaw the review of this article
Chee Jian Pua and Jaydutt Bhalshankar are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 199 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pua, C.J., Bhalshankar, J., Miao, K. et al. Development of a Comprehensive Sequencing Assay for Inherited Cardiac Condition Genes. J. of Cardiovasc. Trans. Res. 9, 3–11 (2016). https://doi.org/10.1007/s12265-016-9673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-016-9673-5