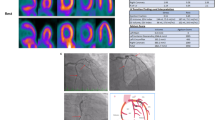
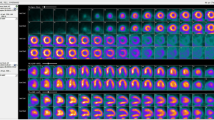
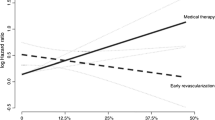
Abstract
Several imaging techniques have been used to assess cardiac structure and function, to understand pathophysiology, and to guide clinical decision making in the setting of acute coronary syndromes (ACS). Over the last years, cardiac positron emission tomography (PET) has affirmed its role in this setting. Indeed, the combined quantitative assessment of myocardial metabolism and perfusion has allowed to better understand the functional status of infarcted and non-infarcted myocardium, thus improving our knowledge of myocardial response to necrosis. More recently, several studies, taking advantage of previous observations in patients with cancer, have shown that PET could also provide important information on the mechanisms of vascular instability through the early identification of activated inflammatory cells in the atherosclerotic plaque. These findings are opening the way to more effective forms of prevention of acute vascular syndromes in high-risk patients; furthermore, new more sensitive and specific tracers for the identification of vascular inflammation are under development. In this review, we describe the potential and limitations of PET in the assessment of ACS.






Similar content being viewed by others
Abbreviations
- ACS:
-
Acute coronary syndrome
- CAD:
-
Coronary artery disease
- CFR:
-
Coronary flow reserve
- FDG:
-
Fluorodeoxyglucose
- FFA:
-
Free fatty acids
- MBF:
-
Myocardial blood flow
- PCI:
-
Percutaneous coronary intervention
- PET:
-
Positron emission tomography
- SPECT:
-
Single-photon emission tomography
- STEMI:
-
ST-elevation acute myocardial infarction
References
Van de Werf, F., Bax, J., Betriu, A., ESC Committee for Practice Guidelines (CPG), et al. (2008). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. European Heart Journal, 29(23), 2909–2945.
Fox, K. A., Dabbous, O. H., Goldberg, R. J., et al. (2006). Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ, 333(7578), 1091.
Galiuto, L., DeMaria, A. N., & Iliceto, S. (2000). Microvascular damage during myocardial ischemia–reperfusion: pathophysiology, clinical implications and potential therapeutic approach evaluated by myocardial contrast echocardiography. Italian Heart Journal, 1(2), 108–116.
Ito, H., Maruyama, A., Iwakura, K., et al. (1996). Clinical implications of the no-reflow phenomenon. A predictor of complications and left ventricular remodelling in reperfused anterior wall myocardial infarction. Circulation, 93, 223–228.
Phelps, M. E., Mazziotta, J. C., & Schelbert, H. R. (Eds.). (1986). Positron emission tomography and autoradiography: principles and applications for the brain and the heart. New York: Raven Press.
Rahmim, A., & Zaidi, H. (2008). PET versus SPECT: strengths, limitations and challenges. Nuclear Medicine Communications, 29, 193–207.
Anagnostopoulos, C., Almonacid, A., El Fakhri, G., et al. (2008). Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. European Journal of Nuclear Medicine and Molecular Imaging, 35(9), 1593–1601.
Di Carli, M. F., & Lipton, M. J. (2007). Cardiac PET and PET/CT imaging editors. New York: Springer.
Stanley, W. C., Recchia, F. A., & Lopaschuk, G. D. (2005). Myocardial substrate metabolism in the normal and failing heart. Physiological Reviews, 85(3), 1093–1129.
Brown, M. A., Myears, D. W., & Bergmann, S. R. (1988). Noninvasive assessment of canine myocardial oxidative metabolism with carbon-11 acetate and positron emission tomography. Journal of the American College of Cardiology, 12, 1054–1063.
Brown, M. A., Myears, D. W., & Bergmann, S. R. (1989). Validity of estimates of myocardial oxidative metabolism with carbon-11 acetate and positron emission tomography despite altered patterns of substrate utilization. Journal of Nuclear Medicine, 30, 187–193.
Camici, P., Ferrannini, E., & Opie, L. H. (1989). Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Progress in Cardiovascular Diseases, 32, 217–238.
Gerber, B. L., Ordoubadi, F. F., Wijns, W., et al. (2001). Positron emission tomography using (18)F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European Community Concerted Action Multicenter study on use of (18)F-fluoro-deoxyglucose positron emission tomography for the detection of myocardial viability. European Heart Journal, 22(18), 1691–1701.
Kaufmann, P. A., & Camici, P. G. (2005). Myocardial blood flow measurement by PET: technical aspects and clinical applications. Journal of Nuclear Medicine, 46(1), 75–88.
Camici, P. G., & Crea, F. (2007). Coronary microvascular dysfunction. The New England Journal of Medicine, 356(8), 830–840.
Beltrame, J. F., Crea, F., & Camici, P. (2009). Advances in coronary microvascular dysfunction. Heart, Lung & Circulation, 18(1), 19–27.
Camici, P., Chiriatti, G., Lorenzoni, R., et al. (1991). Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. Journal of the American College of Cardiology, 17, 879–886.
Ishimori, T., Saga, T., Mamede, M., et al. (2002). Increased 18F-FDG uptake in a model of inflammation: concanavalin A-mediated lymphocyte activation. Journal of Nuclear Medicine, 43, 658–663.
Kelloff, G. J., Hoffman, J. M., Johnson, B., et al. (2005). Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clinical Cancer Research, 11, 2785–2808.
Camici, P. G., & Rimoldi, O. E. (2003). Myocardial blood flow in patients with hibernating myocardium. Cardiovascular Research, 57, 302–311.
Bonow RO, Maurer G, Lee KL, et al. ( 2011). For the STICH Trial Investigators, Myocardial Viability and Survival in Ischemic Left Ventricular Dysfunction, The New England Journal of Medicine 364, 1617–25.
Udelson, J. E., Pearte, C. A., Kimmelstiel, C. D., et al. (2011). The Occluded Artery Trial (OAT) Viability Ancillary Study (OAT-NUC): influence of infarct zone viability on left ventricular remodeling after percutaneous coronary intervention versus optimal medical therapy alone. American Heart Journal, 161(3), 611–621.
Niccoli, G., Burzotta, F., Galiuto, L., et al. (2009). Myocardial no-reflow in humans. Journal of the American College of Cardiology, 54(4), 281–292.
Uren, N. G., Crake, T., Lefroy, D. C., et al. (1993). Delayed recovery of coronary resistive vessel function after coronary angioplasty. Journal of the American College of Cardiology, 21(3), 612–621.
Oliva, P. B. (1981). Pathophysiology of acute myocardial infarction. Annals of Internal Medicine, 94(2), 236–250.
Nahrendorf, M., Swirski, F. K., Aikawa, E., et al. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine, 204(12), 3037–3047.
Godino, C., Messa, C., Gianolli, L., et al. (2008). Multifocal, persistent cardiac uptake of [18-F]-fluoro-deoxy-glucose detected by positron emission tomography in patients with acute myocardial infarction. Circulation Journal, 72(11), 1821–1828.
Ogawa, M., Ishino, S., Mukai, T., et al. (2004). 18F-FDG Accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. Journal of Nuclear Medicine, 45, 1245–1250.
Maschauer, S., Prante, O., Hoffmann, M., et al. (2004). Characterization of 18F-FDG uptake in human endothelial cells in vitro. Journal of Nuclear Medicine, 45, 455–460.
Paik, J. Y., Lee, K. H., Ko, B. H., et al. (2005). Nitric oxide stimulates 18F-FDG uptake in human endothelial cells through increased hexokinase activity and GLUT1 expression. Journal of Nuclear Medicine, 46, 365–370.
Buffon, A., Biasucci, L. M., Liuzzo, G., et al. (2002). Widespread coronary inflammation in unstable angina. The New England Journal of Medicine, 347(1), 5–12.
Abbate, A., Bonanno, E., Mauriello, A., et al. (2004). Widespread myocardial inflammation and infarct-related artery patency. Circulation, 110(1), 46–50.
Lancellotti, P., Melon, P. G., de Landsheere, C. M., et al. (1998). The role of early measurement of nitrogen-13 ammonia uptake for predicting recovery after acute myocardial infarction. International Journal of Cardiac Imaging, 14, 261–267.
Hochman, J. S., Lamas, G. A., Buller, C. E., et al. (2006). Occluded artery trial Investigators coronary intervention for persistent occlusion after myocardial infarction. The New England Journal of Medicine, 355(23), 2395–2407.
Ambrose, J. A., Tannenbaum, M. A., & Alexopoulos, D. (1988). Angiographic progression of coronary artery disease and the development of myocardial infarction. Journal of the American College of Cardiology, 12(1), 56–62.
Virmani, R., Kolodgie, F. D., Burke, A. P., et al. (2000). Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 1262–1275.
Mann, J., & Davies, M. J. (1999). Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart, 82(3), 265–268.
Yonetsu, T., Kakuta, T., Lee, T., et al. (2010). Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. European Heart Journal, 31(13), 1608–1615.
Stone, G. W., Maehara, A., Lansky, A. J., for the PROSPECT Investigators, et al. (2011). A prospective natural-history study of coronary atherosclerosis. The New England Journal of Medicine, 364, 226–235.
Glaser, R., Selzer, F., & Faxon, D. P. (2005). Clinical progression of incidental asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation, 111, 143–149.
Spagnoli, L. G., Bonanno, E., Mauriello, A., et al. (2002). Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. Journal of the American College of Cardiology, 40(9), 1579–1588.
Narducci, M. L., Grasselli, A., Biasucci, L. M., et al. (2007). High telomerase activity in neutrophils from unstable coronary plaques. Journal of the American College of Cardiology, 50(25), 2369–2374.
Liuzzo, G., Kopecky, S. L., Frye, R. L., et al. (1999). Perturbation of the T-cell repertoire in patients with unstable angina. Circulation, 100(21), 2135–2139.
Liuzzo, G., Goronzy, J. J., Yang, H., et al. (2000). Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation, 101(25), 2883–2888.
Giubilato, S., Liuzzo, G., Brugaletta, S., et al. (2011). Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. European Heart Journal, 32(10), 1214–1226.
Strauss, H. W., Dunphy, M., & Tokita, N. (2004). Imaging the vulnerable plaque: a scintillating light at the end of the tunnel? Journal of Nuclear Medicine, 45, 1106–1107.
Vallabhajosula, S., Machac, J., Knesaurek, K., et al. (1996). Imaging atherosclerosis macrophage density by positron emission tomography using F-18-fluorodeoxyglucose (FDG). Journal of Nuclear Medicine, 37, 38P.
Davies, J. R., Izquierdo-Garcia, D., Rudd, J. H., et al. (2010). FDG-PET can distinguish inflamed from non-inflamed plaque in an animal model of atherosclerosis. The International Journal of Cardiovascular Imaging, 26(1), 41–48.
Tahara, N., Kai, H., Ishibashi, M., et al. (2006). Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. Journal of the American College of Cardiology, 48, 1825–1831.
Yun, M., Yeh, D., Araujo, L., et al. (2001). F-18 FDG uptake in the large arteries: a new observation. Clinical Nuclear Medicine, 26, 314–319.
Davies, J. R., Rudd, J. H., Fryer, T. D., et al. (2005). Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke, 36(12), 2642–2647.
Tawakol, A., Migrino, R. Q., Hoffmann, U., et al. (2005). Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. Journal of Nuclear Cardiology, 12, 294–301.
Moustafa, R. R., Izquierdo-Garcia, D., Fryer, T. D., et al. (2010). Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: a pilot study. Circulation Cardiovascular Imaging, 3(5), 536–541.
Wykrzykowska, J., Lehman, S., Williams, G., et al. (2009). Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low carbohydrate, high-fat preparation. Journal of Nuclear Medicine, 50, 563–568.
Rogers, I. S., Nasir, K., Figueroa, A. L., et al. (2010). Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovascular Imaging, 3(4), 388–397.
Folco, E. J., Sheikine, Y., Rocha, V. Z., et al. (2011). Hypoxia but not inflammation augments glucose uptake in human macrophages implications for imaging atherosclerosis with (18)fluorine-labeled 2-deoxy-d-glucose positron emission tomography. Journal of the American College of Cardiology, 58(6), 603–614.
Rojas, S., Martin, A., Arranz, M. J., et al. (2007). Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. Journal of Cerebral Blood Flow and Metabolism, 27, 1975–1986.
Fujimura, Y., Hwang, P. M., Trout Iii, H., et al. (2008). Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [3H]PK11195. Atherosclerosis, 201, 108–111.
Pugliese, F., Gaemperli, O., Kinderlerer, A. R., et al. (2010). Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. Journal of the American College of Cardiology, 56(8), 653–661.
Laitinen, I., Marjamaki, P., Nagren, K., et al. (2009). Uptake of inflammatory cell marker [11C]PK11195 into mouse atherosclerotic plaques. European Journal of Nuclear Medicine and Molecular Imaging, 36(1), 73–80.
Chauveau, F., Boutin, H., Van Camp, N., et al. (2008). Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. European Nuclear Medicine Molecular Imaging, 35(12), 2304–2319.
Nahrendorf, M., Zhang, H., Hembrador, S., et al. (2008). Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation, 117(3), 379–387.
Velazquez, E. J., Lee, K. L., Deja, M. A., et al. (2011). Coronary-artery bypass surgery in patients with left ventricular dysfunction. The New England Journal of Medicine, 364(17), 1607–1616.
Cristell N, Cianflone D, Ammirati D et al (2011) High sensitivity C-reactive protein is within normal levels at the very onset of a first ST-segment elevation acute myocardial infarction (FAMI) in 41% of the cases: a multi-ethnic case control study. Journal of the American College of Cardiology (in press)
Di Carli, M. F. (2004). Advances in positron emission tomography. Journal of Nuclear Cardiology, 11, 719–732.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galiuto, L., Paraggio, L., De Caterina, A.R. et al. Positron Emission Tomography in Acute Coronary Syndromes. J. of Cardiovasc. Trans. Res. 5, 11–21 (2012). https://doi.org/10.1007/s12265-011-9332-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9332-9