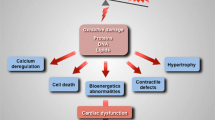
Abstract
Increases in oxidative stress in the heart play an important role in mediating hypertrophy, apoptosis, fibrosis, mitochondrial dysfunction, and the consequent development of heart failure. Although it has been widely believed that electron leakage from the mitochondrial electron transport chain is the primary source of oxidative stress in the failing heart, increasing lines of evidence suggest that enzymes which produce reactive oxygen species may also contribute to it. NADPH oxidases are transmembrane enzymes dedicated to producing superoxide (O2 −) by transferring an electron from NAD(P)H to molecular oxygen. Nox4 is a major NADPH oxidase isoform expressed in the heart. Nox4 is localized primarily at mitochondria in cardiac myocytes, and upregulation of Nox4 hypertrophic stimuli enhances O2 − production, apoptosis, and mitochondrial dysfunction, thereby playing an important role in mediating cardiac dysfunction. Since Nox4 could be a key molecule mediating oxidative stress and pathological hypertrophy, it may serve as an important target of heart failure treatment. In this review, the importance of NADPH oxidases as sources of increased oxidative stress in the failing heart and the role of Nox4 in mediating growth and death of cardiac myocytes are discussed.


Similar content being viewed by others
References
Giordano, F. J. (2005). Oxygen, oxidative stress, hypoxia, and heart failure. Journal of Clinical Investigation, 115(3), 500–508.
Finkel, T. (2003). Oxidant signals and oxidative stress. Current Opinion in Cell Biology, 15(2), 247–254.
Lambeth, J. D. (2004). NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology, 4(3), 181–189.
Bedard, K., & Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews, 87(1), 245–313.
Akki, A., Zhang, M., Murdoch, C., Brewer, A., & Shah, A. M. (2009). NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol, 47(1), 15–22.
Sumimoto, H., Miyano, K., & Takeya, R. (2005). Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochemical and Biophysical Research Communications, 338(1), 677–686.
Brown, D. I., & Griendling, K. K. (2009). Nox proteins in signal transduction. Free Radical Biology and Medicine, 47(9), 1239–1253.
Lyle, A. N., Deshpande, N. N., Taniyama, Y., Seidel-Rogol, B., Pounkova, L., Du, P., et al. (2009). Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation Research, 105(3), 249–259.
Serrander, L., Cartier, L., Bedard, K., Banfi, B., Lardy, B., Plastre, O., et al. (2007). NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochemical Journal, 406(1), 105–114.
Bendall, J. K., Cave, A. C., Heymes, C., Gall, N., & Shah, A. M. (2002). Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation, 105(3), 293–296.
Xiao, L., Pimentel, D. R., Wang, J., Singh, K., Colucci, W. S., & Sawyer, D. B. (2002). Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. American Journal of Physiology Cell Physiology, 282(4), C926–934.
Li, J., Stouffs, M., Serrander, L., Banfi, B., Bettiol, E., Charnay, Y., et al. (2006). The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Molecular Biology of the Cell, 17(9), 3978–3988.
Byrne, J. A., Grieve, D. J., Bendall, J. K., Li, J. M., Gove, C., Lambeth, J. D., et al. (2003). Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circulation Research, 93(9), 802–805.
Kuroda, J., Ago, T., & Sadoshima, J. (2009). Nox4 is a major source of superoxide production in the mouse heart. Circulation, 120(18 Supplement), S614.
Artham, S. M., Lavie, C. J., Milani, R. V., Patel, D. A., Verma, A., & Ventura, H. O. (2009). Clinical impact of left ventricular hypertrophy and implications for regression. Progress in Cardiovascular Diseases, 52(2), 153–167.
Nakamura, K., Fushimi, K., Kouchi, H., Mihara, K., Miyazaki, M., Ohe, T., et al. (1998). Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation, 98(8), 794–799.
Hirotani, S., Otsu, K., Nishida, K., Higuchi, Y., Morita, T., Nakayama, H., et al. (2002). Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation, 105(4), 509–515.
Oka, S., Ago, T., Kitazono, T., Zablocki, D., & Sadoshima, J. (2009). The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. Journal of Molecular Medicine, 87(8), 785–791.
Bush, E. W., & McKinsey, T. A. (2009). Targeting histone deacetylases for heart failure. Expert Opinion on Therapeutic Targets, 13(7), 767–784.
Ago, T., Liu, T., Zhai, P., Chen, W., Li, H., Molkentin, J. D., et al. (2008). A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell, 133(6), 978–993.
Li, J. M., Gall, N. P., Grieve, D. J., Chen, M., & Shah, A. M. (2002). Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension, 40(4), 477–484.
Maytin, M., Siwik, D. A., Ito, M., Xiao, L., Sawyer, D. B., Liao, R., et al. (2004). Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation, 109(9), 1168–1171.
Doerries, C., Grote, K., Hilfiker-Kleiner, D., Luchtefeld, M., Schaefer, A., Holland, S. M., et al. (2007). Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circulation Research, 100(6), 894–903.
Wencker, D., Chandra, M., Nguyen, K., Miao, W., Garantziotis, S., Factor, S. M., et al. (2003). A mechanistic role for cardiac myocyte apoptosis in heart failure. Journal of Clinical Investigation, 111(10), 1497–1504.
Matsuzawa, A., & Ichijo, H. (2005). Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxidants Redox Signaling, 7(3–4), 472–481.
Qin, F., Patel, R., Yan, C., & Liu, W. (2006). NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: Effects of apocynin. Free Radical Biology and Medicine, 40(2), 236–246.
Grishko, V., Pastukh, V., Solodushko, V., Gillespie, M., Azuma, J., & Schaffer, S. (2003). Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: Role of DNA damage. American Journal of Physiology. Heart and Circulatory Physiology, 285(6), H2364–2372.
Ago, T., Kuroda, J., Zhai, P., Fu, C., Li, H., Sadoshima, J. (2010). Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation Research (in press)
Heineke, J., & Molkentin, J. D. (2006). Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature Reviews Molecular Cell Biology, 7(8), 589–600.
Kinnula, V. L., Fattman, C. L., Tan, R. J., & Oury, T. D. (2005). Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. American Journal of Respiratory and Critical Care Medicine, 172(4), 417–422.
Poli, G. (2000). Pathogenesis of liver fibrosis: Role of oxidative stress. Molecular Aspects of Medicine, 21(3), 49–98.
Ha, H., & Lee, H. B. (2003). Reactive oxygen species and matrix remodeling in diabetic kidney. Journal of the American Society of Nephrology, 14(8 Suppl 3), S246–249.
An, S. J., Boyd, R., Zhu, M., Chapman, A., Pimentel, D. R., & Wang, H. D. (2007). NADPH oxidase mediates angiotensin II-induced endothelin-1 expression in vascular adventitial fibroblasts. Cardiovascular Research, 75(4), 702–709.
Johar, S., Cave, A. C., Narayanapanicker, A., Grieve, D. J., & Shah, A. M. (2006). Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB Journal, 20(9), 1546–1548.
Cucoranu, I., Clempus, R., Dikalova, A., Phelan, P. J., Ariyan, S., Dikalov, S., et al. (2005). NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circulation Research, 97(9), 900–907.
Peshavariya, H., Dusting, G. J., Jiang, F., Halmos, L. R., Sobey, C. G., Drummond, G. R., et al. (2009). NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedebergs Archives of Pharmacology, 380(2), 193–204.
Menshikov, M., Plekhanova, O., Cai, H., Chalupsky, K., Parfyonova, Y., Bashtrikov, P., et al. (2006). Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(4), 801–807.
Gorin, Y., Ricono, J. M., Kim, N. H., Bhandari, B., Choudhury, G. G., & Abboud, H. E. (2003). Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. American Journal of Physiology. Renal Physiology, 285(2), F219–229.
Belch, J. J., Bridges, A. B., Scott, N., & Chopra, M. (1991). Oxygen free radicals and congestive heart failure. British Heart Journal, 65(5), 245–248.
Hill, M. F., & Singal, P. K. (1996). Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. American Journal of Pathology, 148(1), 291–300.
Ide, T., Tsutsui, H., Kinugawa, S., Suematsu, N., Hayashidani, S., Ichikawa, K., et al. (2000). Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circulation Research, 86(2), 152–157.
Sam, F., Kerstetter, D. L., Pimental, D. R., Mulukutla, S., Tabaee, A., Bristow, M. R., et al. (2005). Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. Journal of Cardiac Failure, 11(6), 473–480.
Baumer, A. T., Flesch, M., Wang, X., Shen, Q., Feuerstein, G. Z., & Bohm, M. (2000). Antioxidative enzymes in human hearts with idiopathic dilated cardiomyopathy. Journal of Molecular and Cellular Cardiology, 32(1), 121–130.
Tsutsui, H., Ide, T., Hayashidani, S., Suematsu, N., Utsumi, H., Nakamura, R., et al. (2001). Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovascular Research, 49(1), 103–109.
Tsutsui, H. (2006). Mitochondrial oxidative stress and heart failure. Internal Medicine, 45(13), 809–813.
Tsutsui, H., Kinugawa, S., & Matsushima, S. (2009). Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovascular Research, 81(3), 449–456.
Ide, T., Tsutsui, H., Kinugawa, S., Utsumi, H., Kang, D., Hattori, N., et al. (1999). Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circulation Research, 85(4), 357–363.
George, J., & Struthers, A. D. (2008). The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovascular Drug Reviews, 26(1), 59–64.
Bergamini, C., Cicoira, M., Rossi, A., & Vassanelli, C. (2009). Oxidative stress and hyperuricaemia: Pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. European Journal of Heart Failure, 11(5), 444–452.
Landmesser, U., Dikalov, S., Price, S. R., McCann, L., Fukai, T., Holland, S. M., et al. (2003). Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. Journal of Clinical Investigation, 111(8), 1201–1209.
Otani, H. (2009). The role of nitric oxide in myocardial repair and remodeling. Antioxidants Redox Signaling, 11(8), 1913–1928.
Heymes, C., Bendall, J. K., Ratajczak, P., Cave, A. C., Samuel, J. L., Hasenfuss, G., et al. (2003). Increased myocardial NADPH oxidase activity in human heart failure. Journal of the American College of Cardiology, 41(12), 2164–2171.
Nojiri, H., Shimizu, T., Funakoshi, M., Yamaguchi, O., Zhou, H., Kawakami, S., et al. (2006). Oxidative stress causes heart failure with impaired mitochondrial respiration. Journal of Biological Chemistry, 281(44), 33789–33801.
Fu, C., Wu, C., Liu, T., Ago, T., Zhai, P., Sadoshima, J., et al. (2009). Elucidation of thioredoxin target protein networks in mouse. Molecular & Cell Proteomics, 8(7), 1674–1687.
McNally, J. S., Saxena, A., Cai, H., Dikalov, S., & Harrison, D. G. (2005). Regulation of xanthine oxidoreductase protein expression by hydrogen peroxide and calcium. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(8), 1623–1628.
Williams, H. C., & Griendling, K. K. (2007). NADPH oxidase inhibitors: New antihypertensive agents? Journal of Cardiovascular Pharmacology, 50(1), 9–16.
Selemidis, S., Sobey, C. G., Wingler, K., Schmidt, H. H., & Drummond, G. R. (2008). NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacology and Therapeutics, 120(3), 254–291.
Jaquet, V., Scapozza, L., Clark, R. A., Krause, K. H., & Lambeth, J. D. (2009). Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxidants Redox Signaling, 11(10), 2535–2552.
Acknowledgment
The authors thank Daniela Zablocki for critical reading of the manuscript.
Sources of Funding
This work was supported in part by U.S. Public Health Service Grants HL 59139, HL67724, HL69020, HL91469, and AG27211.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuroda, J., Sadoshima, J. NADPH Oxidase and Cardiac Failure. J. of Cardiovasc. Trans. Res. 3, 314–320 (2010). https://doi.org/10.1007/s12265-010-9184-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-010-9184-8