Abstract
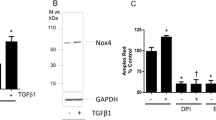
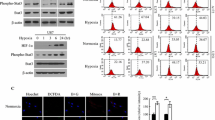
Proliferation and apoptosis of endothelial cells are crucial angiogenic processes that contribute to carcinogenesis and tumor progression. Emerging evidence implicates the regulation of proliferation and apoptosis by reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (H2O2). In the present study, we investigated the roles of the ROS-generating Nox4- and Nox2-containing reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases in proliferation of human endothelial cells by examining the impact of these enzyme systems on (1) specific proliferative and tumorigenic kinases, extracellular regulated kinase1/2 (ERK1/2) and Akt, (2) cytoskeletal organization, and (3) the mechanisms that influence cellular apoptosis. ROS production and the expression of NADPH oxidase subunit Nox4, but not Nox2, were markedly higher in proliferating than in quiescent endothelial cells. Addition of the H2O2 scavenger catalase or downregulation of Nox4 protein with specific siRNA reduced ROS levels, cell proliferation, and ERK1/2 phosphorylation but had no effect on either cell morphology or caspase 3/7 activity. Although downregulation of Nox2 protein with siRNA also reduced ROS production and cell proliferation, it caused an increase in caspase 3/7 activity, reduced Akt phosphorylation, and caused cytoskeletal disorganization. Therefore, in endothelial cells, Nox4-derived H2O2 activates ERK1/2 to promote proliferation, whereas Nox2-containing NADPH oxidase maintains the cytoskeleton and prevents apoptosis to support cell survival. Our study provides a new understanding of the molecular mechanisms that underpin endothelial cell survival and a rationale for the combined suppression of Nox4- and Nox2-containing NADPH oxidases for unwanted angiogenesis in cancer.







Similar content being viewed by others
Abbreviations
- DPI:
-
Diphenylene iodonium
- FAD:
-
Flavin adenine dinucleotide
- FBS:
-
Fetal bovine serum
- H2-DCFDA:
-
Dichlorodihydrofluorescein-diacetate
- HMECs:
-
Human microvascular endothelial cells
- HUVEC:
-
Human umbilical vein endothelial cells
- H2O2 :
-
Hydrogen peroxide
- ERK1/2:
-
Extracellular regulated kinase1/2
- l-NAME:
-
NG-Nitro-l-arginine methyl ester
- 170-ODYA:
-
17-Octadecynoic acid
- ROS:
-
Reactive oxygen species
- PI-3 K:
-
Phosphatidyl inositol 3-kinase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Abid MR, Kachra Z et al (2000) NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486(3):252–256
Alom-Ruiz SP, Anilkumar N et al (2008) Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10(6):1089–1100
Bayraktutan U (2005) Coronary microvascular endothelial cell growth regulates expression of the gene encoding p22-phox. Free Radic Biol Med 39(10):1342–1352
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Brown MR, Miller FJ Jr et al (1999) Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85(6):524–533
Chen JX, Chen Y et al (2004) Dual functional roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am J Physiol Heart Circ Physiol 287(1):H187–H195
Chen JX, Zeng H et al (2006) Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291(4):H1563–H1572
Chen K, Kirber MT et al (2008) Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181(7):1129–1139
Datla SR, Peshavariya H et al (2007) Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol 27(11):2319–2324
Deshpande NN, Sorescu D et al (2002) Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal 4(5):845–854
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5(11):876–885
Griendling KK, Sorescu D et al (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20(10):2175–2183
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Harfouche R, Malak NA et al (2005) Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. Faseb J 19(12):1728–1730
Ikeda S, Yamaoka-Tojo M et al (2005) IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25(11):2295–2300
Irani K (2000) Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 87(3):179–183
Kawahara T, Ritsick D et al (2005) Point Mutations in the Proline-rich Region of p22phox Are Dominant Inhibitors of Nox1- and Nox2-dependent Reactive Oxygen Generation. J Biol Chem 280(36):31859–31869
Kim YM, Kim KE et al (2006) Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res 66(12):6167–6174
Kondrikov D, Han HR et al (2006) Growth and density-dependent regulation of NO synthase by the actin cytoskeleton in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290(1):L41–L50
Li JM, Shah AM (2002) Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277(22):19952–19960
Moldovan L, Moldovan NI et al (2000) Redox changes of cultured endothelial cells and actin dynamics. Circ Res 86(5):549–557
Natarajan V, Pendyala S et al (2008) Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal . doi:10.1089/ARS.2008.2203
Opitz N, Drummond GR et al (2007) The ‘A’s and ‘O’s of NADPH oxidase regulation: a commentary on “Subcellular localization and function of alternatively spliced Noxo1 isoforms”. Free Radic Biol Med 42(2):175–179
Pendyala S, Usatyuk P, Gorshkova IA, Garcia JG, Natarajan V (2008) Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11(4):841–860. doi:10.1089/ars.2008.2231
Peshavariya HM, Dusting GJ et al (2007) Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res 41(6):699–712
Petry A, Djordjevic T et al (2006) NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8(9–10):1473–1484
Rao GN, Berk BC (1992) Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 70(3):593–599
Selemidis S (2008) Suppressing NADPH oxidase-dependent oxidative stress in the vasculature with nitric oxide donors. Clin Exp Pharmacol Physiol 35(11):1395–1401
Selemidis S, Dusting GJ et al (2007) Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res 75(2):349–358
Selemidis S, Sobey CG et al (2008) NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol Ther 120(3):254–291
Stone JR, Collins T (2002) The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 9(4):231–238
Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9(6):731–739
Ushio-Fukai M, Tang Y et al (2002) Novel role of gp91(phox)-containing NAD(P) H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91(12):1160–1167
Van Buul JD, Fernandez-Borja M et al (2005) Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7(3–4):308–317
Xia C, Meng Q et al (2007) Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67(22):10823–10830
Zanetti M, Zwacka R et al (2001) Superoxide anions and endothelial cell proliferation in normoglycemia and hyperglycemia. Arterioscler Thromb Vasc Biol 21(2):195–200
Zanetti M, Katusic ZS et al (2002) Adenoviral-mediated overexpression of catalase inhibits endothelial cell proliferation. Am J Physiol Heart Circ Physiol 283(6):H2620–H2626
Acknowledgments
Supported by the National Health and Medical Research Council (NHMRC) of Australia. Fellowships from the NHMRC supported HP (327401), GJD (400303), GRD (465109), CGS (350327) and SS (232324).
Author disclosure statement
There are no competing financial interests for all authors involved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peshavariya, H., Dusting, G.J., Jiang, F. et al. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmied Arch Pharmacol 380, 193–204 (2009). https://doi.org/10.1007/s00210-009-0413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-009-0413-0