Abstract
Light is a powerful environmental factor influencing diverse brain functions. Clinical evidence supports the beneficial effect of light therapy on several diseases, including depression, cognitive dysfunction, chronic pain, and sleep disorders. However, the precise mechanisms underlying the effects of light therapy are still not well understood. In this review, we critically evaluate current clinical evidence showing the beneficial effects of light therapy on diseases. In addition, we introduce the research progress regarding the neural circuit mechanisms underlying the modulatory effects of light on brain functions, including mood, memory, pain perception, sleep, circadian rhythm, brain development, and metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in lighting conditions have broad effects on diverse physiological and behavioral functions, including circadian rhythm, mood, and cognition [1, 2]. In humans, light therapy can alleviate depression, promote cognitive function, and relieve pain symptoms [3,4,5,6,7,8,9,10,11,12]. It provides a solution for the treatment of brain diseases with the advantages of non-invasiveness, few side-effects, and low cost. Given the acknowledgment of its beneficial effects on brain diseases, the neural mechanisms underlying the beneficial effects of light therapy on brain functions are not well understood. This has caused obstacles to the application and optimization of light therapy in the clinic. Here, we review the progress of human clinical research into light therapy and introduce the basic research progress regarding the circuit mechanisms underlying the modulatory effects of light on brain functions. Through analyzing these research findings, we hope to provide readers with a comprehensive understanding of the modulatory effects of light on brain functions and inspire new ideas for the application and optimization of light therapy.
Clinical Evidence for Light Therapy
Light Therapy for Depression
As early as the 1980s, light therapy was first used to treat seasonal affective disorder (SAD) in clinical settings. The results of a meta-analysis that included 19 randomized controlled trials confirmed the effectiveness of light therapy in the treatment of SAD (d = −0.37, 95% CI: −0.63, −0.12) [13]. As a cost-effective physical intervention, light therapy has many advantages, such as high safety, mild side-effects, and convenience. To date, light therapy has become the first-line treatment for SAD. In addition to SAD, an increasing number of clinical studies have indicated that light therapy has certain therapeutic effects on various types of non-seasonal depression. For instance, Lam and colleagues conducted a randomized controlled trial on 122 patients with major depressive disorder, and the results showed that the light therapy group (d = 0.80, 95% CI: 0.28, 1.31) and the combined treatment group (light therapy/fluoxetine) (d = 1.11, 95% CI: 0.54, 1.64) had significantly superior improvements in outcomes compared to those in the placebo group [14]. Pierre and colleagues conducted a systematic review of major depressive disorder and concluded that light therapy and antidepressants were equally effective (SMD = 0.19, 95% CI: −0.08, 0.45) and that light therapy combined with antidepressants was significantly better than monotherapy with antidepressants (SMD = 0.56, 95% CI: 0.24, 0.88) [15]. An open trial suggested that 3 weeks of bright light therapy is efficacious for antepartum depression, and the benefits were seen at the 2-week follow-up assessment [16]. Our previous study also suggested that 8 weeks of bright light therapy had beneficial effects in treating subthreshold depression in a college student sample [17]. In summary, the beneficial effects of light therapy have been confirmed in several types of depression, but the optimal parameters for this intervention are controversial. However, guidelines for the clinical applications of light therapy in depression are lacking.
Light Therapy for Cognition
Light is essential for many cognitive tasks. A Cochrane meta-study that included three randomized controlled trials and two before-after trials with 282 daytime worker participants, showed that bright illumination in the environment improves alertness [18]. Our recent meta-study showed that light exposure in laboratory settings improves both objective and subjective alertness, and the correlated color temperature of light treatment is a key parameter to determine the beneficial effects [19]. The potential application of light therapy for cognitive disorders has been increasingly recognized in the last decade [20]. A recent randomized placebo-controlled trial of blue wavelength exposure was conducted in 32 adults with mild traumatic brain injury [21]. Compared with the placebo, blue light led to reduced daytime sleepiness and improved executive functioning, which was associated with brain changes in the retinohypothalamic system [21]. A Cochrane meta-study found no adequate evidence of the effectiveness of light therapy in managing cognitive function in dementia [22]. A recent study using a crossover design suggested that a whole-day lighting scheme that follows the natural light/dark cycle improves cognitive performance in older adults, as indexed by the trail-making test (assessing executive function) and the digit symbol substitution test (assessing processing speed and attention) [23]. Another recent clinical trial demonstrated the efficacy of bright light therapy in improving objective cognitive function in the survivors of hematopoietic stem cell transplants [24]. Blue-enriched light therapy has been found to be beneficial in improving general cognitive function in patients with mild and moderate Alzheimer’s disease (AD) [25]. Overall, it seems that light intervention is useful for improving alertness in healthy individuals, but the evidence for its effect on other cognitive functions, such as memory, attention, and executive function, is not adequate. Investigations on the effectiveness of light therapy in patients with cognitive disorders are scarce, and conflicting results have been reported. We speculate that at least three reasons contribute to these inconsistent results. First, the stage and severity of cognitive impairment should be considered. Second, outcomes should be expanded to many cognitive domains, rather than general cognition. Finally, light therapy may be a good supplementary intervention to traditional cognitive interventions. In the future, more studies are required to investigate the efficacy of light therapy on different aspects of cognition.
Light Therapy for Pain
In clinical practice, the common approaches to treating chronic pain include opioid analgesic medication [26], cognitive behavioral therapy [27, 28], meditation, and acupuncture [29, 30]. However, these approaches have various limitations. For instance, pain treatments are not always effective, have side-effects, and are expensive [31]. Although clinical evidence documenting the relationship between light therapy and pain is scarce, light therapy is a promising, available, and safe intervention to manage chronic pain. A prospective study evaluated the effect of natural sunlight on pain among patients undergoing spinal surgery [9]. The results showed that patients staying on the bright side of the room had decreased pain, analgesic medication use, and pain medication cost than those staying on the dim side [9]. Patients with fibromyalgia and US military veterans often report experiencing pain; Burgess and colleagues conducted three studies on the effect of bright light therapy in these two populations. Their first study indicated that bright light therapy improves pain sensitivity (less sensitive to pain) in female patients with fibromyalgia [10]. The second study suggested that morning bright light therapy reduces pain intensity, pain behavior, and the thermal pain threshold in US veterans with chronic low back pain [11]. Importantly, phase advances in circadian timing were significantly associated with increased pain tolerance in both studies. A further study evaluated volatility and showed that bright light therapy was related to participants experiencing fewer “pain flares” [32]. Overall, several limitations, such as small sample sizes and the lack of a double-blind design, were noted for the above studies. More future clinical studies of high quality are needed to document the evidence for light therapy and pain.
Light Therapy for Sleep and Circadian Rhythms
Light, sleep, and circadian rhythms have closely interacted to help organisms to adapt to the environment [2]. First, light influences the suprachiasmatic nucleus (SCN) that controls circadian rhythms. Second, light suppresses melatonin secretion, which is crucial for the regulation of circadian rhythms and sleep. Consequently, light therapy has been applied as a potential treatment for sleep disorders. For instance, a randomized control study suggested that light and dark exposure in shift work nurses improves their insomnia symptoms [33]. Morning bright light exposure advances the circadian rhythms in patients with insomnia [34] and delays sleep phase syndrome [35]. However, one study reported limited effects of light therapy on individuals >55 years old with mild early-morning awakenings [36]. A meta-study that included 53 studies and 1154 participants found beneficial effects of light therapy on sleep problems in general, including insomnia, and the sleep problems associated with AD and dementia, but limited effects on circadian rhythm sleep disorders [37]. Furthermore, combined bright light and melatonin therapies have been shown to be superior to single therapy in advancing the phase in healthy individuals and improving sleep outcomes in elderly populations with cognitive decline [38]. Light therapy also improves sleep quality in patients with Parkinson’s disease [39]. It has been noted that the parameters of light therapy greatly influence the sleep outcomes and circadian rhythms. A consistent finding is that light exposure during daytime has beneficial effects on sleep, whereas light exposure at night has adverse effects. More investigations are needed to explore the effects of light characteristics on sleep and circadian rhythms.
Human Neuroimaging Studies Related to Light Therapy
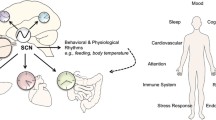
Although light therapy has been widely reported to be beneficial in clinical studies (Fig. 1), the underlying mechanisms remain largely unknown. The clinical practice of light therapy in the treatment of depression is mainly based on the phase-shifting hypothesis, which indicates that light has a role in re-synchronizing circadian rhythms [40]. However, other mechanisms may also mediate the antidepressant effect of light therapy based on three lines of evidence. First, light therapy requires a higher intensity (>5000 lx) to treat depression than to alter circadian rhythms (~120 lx). Second, the relief of depressive symptoms is not consistently associated with changes in circadian rhythm [40, 41]. Finally, light therapy has been shown to be efficacious in treating not only seasonal depression but also many types of non-seasonal depression [14, 42,43,44,45]. Pertinently, not all types of non-seasonal depression are related to phase shifting.
Neuroimaging techniques have provided some clues for the neural mechanisms underlying the beneficial effects of light therapy. An early study collected functional magnetic resonance imaging (fMRI) data while participants attended to auditory and visual stimuli in darkness following short-term light exposures in the scanner [46]. The results revealed increased activation in the occipito-parietal network and decreased activation in the hypothalamus [46]. By using an auditory oddball task, another fMRI study found that short-term exposure to bright white light induced dynamic responses in the posterior thalamus and subcortical regions supporting attentional effects [47]. A further fMRI study evaluated the wavelength effect of light exposure on an auditory working memory task [48]. Compared with green light exposure, blue light exposure led to changes in the left intraparietal sulcus, supramarginal gyrus, right insula, left middle frontal gyrus, and left thalamus [48]. All of the above studies demonstrated non-visual responses to short-term light exposure in the human brain within scanners. However, neuroimaging studies on light therapy are few. We only identified two relevant studies. Fisher and colleagues conducted an fMRI study on healthy individuals with an emotional faces paradigm [49]. The results demonstrated that a three-week bright-light intervention changed amygdala-prefrontal reactivity and functional coupling, which was partly moderated by the 5-HTTLPR (serotonin-transporter-linked promoter region) genotype [49]. This important finding has provided the first evidence indicating that serotonin and the threat-related circuit may underlie the effects of light therapy in humans. Another study used resting-state fMRI to investigate the neural mechanisms underlying two-week morning bright light exposure in individuals with sleep disturbances [50]. The results demonstrated decreased functional connectivity in the anterior insular and frontal opercular regions that were correlated with decreased sleep latency in a bright light group [50]. More longitudinal neuroimaging studies are needed to reveal the neural mechanisms underlying the non-visual effects of light.
Animal Studies
As noted above, clinical studies of light therapy have mainly focused on the utility of this treatment for mood-, cognition-, pain-, and sleep-related disorders. Due to the complexity of the biological mechanisms underlying these disorders and the limitations of technologies that can be used in humans, the neural mechanisms of light therapy are poorly understood. To further analyze the neural mechanism of light therapy, experimental animals are indispensable research subjects. Unveiling the neuronal basis for the effects of light on brain functions in animal models constitutes a promising step toward new treatments for neuropsychiatric disorders.
Photosensitive Cells in the Retina
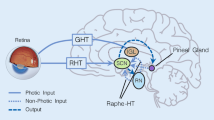
In mammals, the effects of external light on brain functions are mainly mediated by specific visual circuits [51]. At the beginning of the visual circuits, photosensitive cells in the retina convert light information into bioelectrical information that is transmitted to different brain regions [51,52,53]. In the mammalian retina, there are three types of light-sensitive cells: rods, cones, and intrinsic photosensitive retinal ganglion cells (ipRGCs) [54, 55]. Among these, rods and cones are conventional photoreceptors that transmit light signals to RGCs through bipolar cells. Owing to the expression of the photosensitive protein melanopsin, ipRGCs not only receive light signals transmitted from conventional photoreceptors but also respond directly to external light stimuli even in the absence of rods and cones [55,56,57,58,59,60]. Studies have found that ipRGCs are highly conserved in several species, including humans [52, 61, 62], and they transmit light information to multiple brain regions to regulate brain functions unrelated to image-forming vision, including the circadian rhythm [1], cognition [20], and mood [2]. The discovery of ipRGCs also opened up a new field of research, namely, the non-image-forming visual functions of light.
IpRGCs can be divided into different subtypes according to their morphological characteristics, such as the size of the somata, the ramification pattern of dendrites stratified in the inner plexiform layer of the retina, and the size and complexity of the dendritic field. At present, it is known that mouse ipRGCs are divided into six subtypes, M1-M6 [52]; rat ipRGCs are divided into five subtypes, M1-M5 [61], and human ipRGCs are known to have four subtypes, M1-M4 [62]. In addition to morphological differences, ipRGCs vary in other aspects. For example, M1-ipRGCs have the highest level of melanopsin expression, while M4-ipRGCs express melanopsin at a very low level [63, 64]. In addition, different subtypes of ipRGC differ in their response to light and their projection patterns to brain regions [61, 65, 66] (Fig. 2). It is precisely because of the cellular heterogeneity of ipRGCs that they mediate the effects of light on diverse brain functions, such as mood, memory, pain perception, sleep, circadian rhythm, brain development, and metabolism.
The efferent projections of ipRGCs. The main brain targets of ipRGCs. IPL, inner plexiform layer; ipRGCs, intrinsic photosensitive retinal ganglion cells; AH, anterior hypothalamus; BST, bed nucleus of the stria terminalis; SPZ, subparaventricular zone; VLPO, ventrolateral preoptic area; SON, supraoptic nucleus; SCN, suprachiasmatic nucleus; MA, medial amygdaloid nucleus; pHb, perihabenular nucleus; LHb, lateral habenula; SC, superior colliculus; PAG, periaqueductal gray; OPN, olivary pretectal nucleus; dLGN, dorsal lateral geniculate nucleus; IGL, intergeniculate leaflet; vLGN, ventral lateral geniculate nucleus.
Circuit Mechanisms Underlying the Effects of Light on Mood
Clinical studies of SAD patients have found that rapid tryptophan depletion reverses the antidepressant effects of light therapy [67,68,69], suggesting that light information might influence depressive-like behaviors through specific circuits linking the retina and the midbrain monoaminergic centers. The lateral habenula (LHb), part of the epithalamus, is a highly conserved nucleus across species, and it regulates the flow of information from the limbic system to the midbrain monoaminergic centers [70]. Studies of rodents have suggested that light influences neural activity in the LHb [2, 71]. Therefore, visual circuits related to the LHb might mediate the effects of light on depressive-like behaviors. Consistent with this view, our recent study of mice found that the ventral lateral geniculate nucleus and intergeniculate leaflet (vLGN/IGL) are important relay regions linking the retina and LHb [72]. Bright light signals transmitted by a subset of M4-ipRGCs activate a subset of GABAergic neurons in the vLGN/IGL, which in turn inhibit the activity of excitatory neurons in the LHb. We found that specific activation of RGCs projecting to the vLGN/IGL, activation of LHb-projecting vLGN/IGL neurons, or inhibition of postsynaptic LHb neurons is sufficient to decrease the depressive-like behaviors evoked by long-term exposure to aversive stimuli or chronic social defeat stress. Furthermore, we demonstrated that the activation of the retina-vLGN/IGL-LHb pathway is needed for the anti-depressive effects of bright light treatment [72]. These results provide a potential mechanistic explanation for the antidepressant effects of bright light treatment.
Unlike the role of the LHb in mediating the beneficial effects of light on depressive-like behaviors, recent studies have shown that the perihabenular nucleus (pHb) located in the dorsal thalamus mediates the negative effects of light on depressive-like behaviors [73, 74]. For example, Fernandez and colleagues found that long-term exposure to an ultradian light : dark cycle (T7 cycle : 3.5 h light and 3.5 h dark) accompanied by pHb activation increases depressive-like behaviors in mice [73]. They further demonstrated that the depression-inducing effects of a fast ultradian light cycle are mediated by an M1-ipRGC→pHb→mPFC pathway. In addition to the ultradian light/dark cycle, An and colleagues found that excessive light exposure at night (LAN) also increases depressive-like behaviors without disrupting the circadian rhythm [74]. Importantly, An and colleagues demonstrated that the depression-inducing effects of LAN are mediated by a visual circuit consisting of M1-ipRGCs, the pHb, and the nucleus accumbens. Those two studies provide direct evidence that the pHb-related visual circuits play a pivotal role in mediating the negative effects of light on depressive-like behaviors.
However, the relevant question is about the idea that plenty of light during the daytime should also activate the M1-ipRGC→pHb pathway, which can increase depressive-like behaviors. Why does daytime light exposure (such as bright light therapy) have an antidepressant effect? One possible explanation is that the excitability of pHb neurons is higher at night than in the daytime and tends to conduct nighttime light information [74]. Taken together, recent studies conducted in rodents have suggested that the pHb acts as a valve controlled by the circadian rhythm, specifically mediating the regulation of negative mood by nocturnal light messages, and daytime light exposure alleviates depression through the LHb-related visual circuits (Fig. 3A).
Circuit mechanisms underlying the effects of light treatment. A Bright light treatment (LT) in the daytime induces antidepressant effects through the M4 ipRGC→vLGN/IGL→LHb pathway (orange), while light at night (LAN) increases depressive-like behaviors through the M1 ipRGC→pHb→NAc pathway (blue). B LT promotes spatial memory through the M4 ipRGC→vLGN/IGL→Re pathway (orange), while a fast ultradian light cycle (T7, 3.5 h light, and 3.5 h dark) impairs memory (blue) through the M1 ipRGC→SCN pathway. (C) LT exerts antinociceptive effects through the M4 ipRGC→vLGN/IGL→v/lPAG pathway, while green light treatment (GLT) has antinociceptive effects through the cRGC→vLGN→DRN pathway and V2M→ACC pathway. RGC, retinal ganglion cell; vLGN/IGL, ventral lateral geniculate nucleus and intergeniculate leaflet; PHb, perihabenular nucleus; LHb, lateral habenula; NAc, nucleus accumbens; SCN, suprachiasmatic nucleus; Re, reunions nucleus; v/lPAG, lateral and ventral lateral periaqueductal gray; DRN, dorsal raphe; V2M, the secondary visual cortex; ACC, anterior cingulate cortex.
Circuit Mechanisms Underlying the Effect of Light on Memory
Memory dysfunction is a typical characteristic of AD, the pathogenesis of which is complex, including amyloid-β (Aβ) deposition, tau accumulation, and disrupted network oscillation [75, 76]. Studies conducted in mice have shown that light flickering at 40 Hz (gamma entrainment using sensory, GENUS) drives gamma oscillations in the visual cortex [77]. The authors demonstrated that the long-term application of GENUS improves memory in several mouse models of AD, including the CK-p25, P301S, and 5xFAD models [78]. In addition, by training neurons to oscillate at gamma frequency, GENUS triggers the engulfment activity of microglia, while decreasing Aβ deposition and tau accumulation in several brain regions, including the visual cortex, hippocampus (HPC), and prefrontal cortex (PFC) [77, 78]. Moreover, GENUS enhances synaptic function in neurons [78]. These findings uncover a previously unappreciated function of gamma rhythms in recruiting both neural and glial responses to attenuate AD-associated pathology. However, the circuit mechanisms underlying the effects of GENUS on memory are still unknown. It should also be noted that a recent study found that 40 Hz flickering light does not entrain gamma oscillations and suppress Aβ in the brains of APP/PS1 and 5xFAD mouse models of AD [79]. Thus, more detailed work is needed to investigate the effects of GENUS and the underlying mechanism.
Accumulating evidence suggests that bright light has beneficial effects on memory. In humans, brighter illumination during the day improves cognitive performance [4,5,6,7,8], and bright light therapy appears to attenuate cognitive deterioration in early-stage dementia [22, 80]. In rodents, bright light has been shown to enhance fear and spatial memory [81,82,83]. However, the neural mechanisms underlying the effects of bright light on memory are not well understood. A recent study by our group probed the circuit mechanisms underlying the beneficial effects of bright light treatment on spatial memory [84]. We found that long-term exposure to bright light promotes the spatial memory tested in the novel location test and Morris water maze test in young WT mice (3 months old); this is accompanied by the increased power of gamma oscillation in the HPC during the performance of a spatial memory-related task [84]. These results suggest that bright light treatment might promote spatial memory through certain visual circuits associated with the HPC. However, it is well established that the HPC does not receive direct retinal projections. To unveil the neural circuits underlying the beneficial effects of bright light on spatial memory, we conducted whole-brain c-Fos mapping to determine the neural substrates that can be regulated by bright light. We found that bright light increases c-Fos expression in the nucleus reunions (Re) [84], which interacts with the HPC and plays an important role in the regulation of memory [85,86,87]. These results suggest that the spatial-memory-promoting effects of bright light might be mediated by a visual circuit linking the retina and Re. Consistent with this view, we found that bright light promotes spatial memory through activating an M4-ipRGC→vLGN/IGL→Re pathway [84] (Fig. 3B). These results reveal a dedicated subcortical visual circuit that mediates the spatial-memory-promoting effects of bright light treatment.
It should be noted that both GENUS and bright light promote spatial memory and increase gamma oscillation in the HPC. One might expect that bright light should also improve spatial memory in a mouse model of AD. However, we found that long-term exposure to bright light does not significantly promote spatial memory in 6-month-old 5xFAD mice [84]. In addition, unlike GENUS, bright light alone does not directly increase gamma oscillation in the HPC [84]. The HPC gamma oscillation detected during the spatial memory-related task may be too weak to reduce the amyloid load and reverse the deficits in spatial memory of 5xFAD mice. On the other hand, GENUS does not show beneficial effects on spatial memory in WT mice [78], suggesting that the neural mechanisms underlying the spatial-memory-promoting effects of bright light treatment and GENUS are different.
In addition to the beneficial effects of light on memory, certain light patterns can impair memory. Fernandez and colleagues found that a fast ultradian light cycle (T7) not only increases depressive-like behaviors but also results in memory deficits, accompanied by decreased cellular plasticity in the HPC [73]. They demonstrated that the negative effects of the fast ultradian light cycle on memory are mediated by an M1-ipRGC→SCN pathway (Fig. 3B). Given that the SCN plays a pivotal role in the regulation of circadian rhythm, these results suggest that a disrupted circadian rhythm might underlie fast ultradian light cycle-induced memory deficits. In addition, studies conducted in grass rats, a diurnal rodent species, found that dim light exposure (5 lx) at night impairs spatial memory accompanied by a decreased dendritic length in the dentate gyrus and basilar CA1 dendrites [88], without affecting the circadian rhythm tested in the wheel-running test. This result suggests that certain visual circuits unrelated to the regulation of circadian rhythm might also mediate the negative effects of LAN on memory.
Circuit Mechanisms Underlying the Effect of Light on Pain Perception
Clinical studies have shown that light therapy can relieve pain symptoms in patients with chronic low back pain, headache, fibromyalgia, and postoperative pain [9,10,11,12]. Consistent with this, animal studies have shown that light also has antinociceptive effects in rodents [89, 90]. However, the precise circuits that mediate the effects of light on nocifensive behaviors remain unclear. Our recent study in mice found that GABAergic neurons in the vLGN/IGL directly synapse with GABAergic neurons in the lateral and ventral lateral periaqueductal gray (l/vlPAG) [91], which is an important part of the ascending pain conduction and descending pain regulation systems [92,93,94,95]. We found that specific activation of the vLGN/IGL not only induces inhibitory postsynaptic currents in l/vlPAG GABAergic neurons but also reduces the excitatory effects of pain-related stimuli on l/vlPAG GABAergic neurons [91], suggesting that the vLGN/IGL→l/vlPAG pathway might modulate pain-related behaviors. Consistent with this, we found that specific activation of l/vlPAG-projecting vLGN/IGL neurons or inhibition of l/vlPAG postsynaptic neurons not only elevates the pain threshold in WT mice but also improves the pain-related symptoms in mouse models of pain [91], indicating the antinociceptive effects induced by activation of the vLGN/IGL→l/vlPAG pathway. We further found that the vLGN/IGL→l/vlPAG pathway receives direct innervation from RGCs [91], suggesting that light information transmitted by the retina→vLGN/IGL→l/vlPAG pathway might modulate pain-related behaviors. In support of this view, we demonstrated that the antinociceptive effects of bright light treatment are dependent on the activation of the retina→vLGN/IGL→l/vlPAG pathway [91] (Fig. 3C).
In addition to bright light, exposure to green light also has antinociceptive effects in both humans and rodents [89, 96,97,98]. A recent study in mice found that activation of the retina→vLGN→dorsal raphe (DRN) pathway is needed for the antinociceptive effects of green light [99]. It is worth noting that conventional photoreceptors, but not ipRGCs, are required for the antinociceptive effects of green light, suggesting that conventional RGCs can also mediate the antinociceptive effects of light (Fig. 3C). Interestingly, another study revealed that a circuit linking the visual cortex and the anterior cingulate cortex circuit plays a pivotal role in mediating the antinociceptive effects of green light (Fig. 3C) [100]. Given that the vLGN-related visual circuits are also needed for green light analgesia, it would be interesting to investigate the mechanisms underlying the interaction between vLGN-related visual circuits and the cerebral cortex.
Circuit Mechanisms Underlying the Effects of Light on Sleep and Circadian Rhythms
Sleep is regulated by both homeostatic mechanisms and circadian rhythm [101]. Light can modulate sleep by entraining the circadian rhythm [2]. The SCN located in the hypothalamus is the central pacemaker of the circadian timing system [102]. Since ipRGCs project directly to the SCN, it was assumed that ipRGCs play a major role in mediating the effects of light on circadian rhythms. Consistent with this view, it has been found that specific ablation of ipRGCs results in the complete loss of circadian photoentrainment [103,104,105,106]. However, abolishing the intrinsic sensitivity to light of ipRGCs by removing the gene encoding the photosensitive protein melanopsin does not significantly affect circadian photoentrainment [107, 108]. Given that ipRGCs also receive light signals transmitted by rods and cones, it is plausible that rods/cones phototransduction via ipRGCs is sufficient for circadian photoentrainment. In support of this hypothesis, studies have shown that light does not regulate the circadian rhythm tested in the wheel-running test in mice lacking both rods/cones and melanopsin [109].
In addition to the SCN, other brain regions can also mediate the effects of light on sleep. It has been reported that sleep-related brain regions, such as the ventrolateral preoptic area (VLPO) and the superior colliculus (SC), are directly innervated by ipRGCs [66], and the changed neural activity in the VLPO and SC might mediate the acute light-induced sleep at night in mice [110, 111]. In addition, Zhang and colleagues found that acute dark exposure during the daytime induces wakefulness in mice by activating a retina→SC→VTA pathway [112]. They demonstrated that a dark pulse administered during the daytime disinhibits VTA dopaminergic neurons by inhibiting SC GABAergic neurons, which consequently leads to increased wakefulness and reduced sleep [112]. Thus, both circadian-related and circadian-unrelated mechanisms underlie the modulatory effects of light on sleep.
Circuit Mechanisms Underlying the Effects of Light on Brain Development
The light sensation is crucial for brain development [113]. For example, raising animals in complete darkness or depriving one or both eyes at early developmental stages leads to significant shrinkage of thalamic axonal arbors in the visual cortex [114]. In 2014, Yu and colleagues found that dark rearing from birth reduces the excitatory synaptic transmission in multiple sensory cortices, and this impairment can be rescued by elevating the release of oxytocin from the paraventricular nucleus (PVN) through an enriched environment during the early days of dark rearing [115]. These findings suggest that the neuropeptide oxytocin is a key molecule in mediating the effects of light on brain development. However, the visual circuits that mediate the effects of light on oxytocin secretion still need to be determined. It is well established that ipRGCs become light sensitive much earlier than rods and cones, and play a pivotal role in mediating the earliest light sensation in mammals [116]. This suggests that visual circuits associated with ipRGCs might be important for mediating the effects of light on brain development. Consistent with this, a recent study by Hu and colleagues found the ipRGC→supraoptic nucleus (SON)→PVN pathway mediates light-promoted brain development [117]. They demonstrated that light-induced activation of ipRGCs increases the release of oxytocin from the SON and PVN into the cerebrospinal fluid [117]. Importantly, they found that a lack of ipRGC-mediated, light-promoted early cortical synaptogenesis compromises learning ability in adult mice [117]. These results provide new insight into the circuit mechanisms of the impacts of light on brain development.
In addition to the development of learning, early light sensation also influences non-photic circadian entrainment. Fernandez and colleagues established a time-restricted feeding model in mice by limiting food access to a 7-h period (ZT4-ZT11) [118]. They found that the innervation of ipRGCs at early postnatal stages influences IGL neurons that express neuropeptide Y, guiding the assembly of a functional IGL→SCN pathway [118]. Furthermore, they demonstrated that ablation of ipRGCs during early postnatal stages alters the connectivity between the IGL and SCN, reduces the expression of neuropeptide Y in the SCN, and results in decreased food-anticipatory activity in adult mice [118]. This result provides direct evidence that light sensation in the early postnatal period affects entrainment to time-restricted feeding through the ipRGC→IGL→SCN pathway.
Circuit Mechanism Underlying the Effects of Light on Metabolism
Public health studies have shown that a disrupted circadian rhythm is highly relevant to metabolic diseases, including obesity [119, 120], diabetes [121], and cardiovascular disease [122]. Animal studies have also found that a circadian rhythm disrupted by varying light patterns affects metabolism [123, 124]. For example, long-term exposure to light at night alters internal hormonal rhythms [125,126,127] and thereby influences glucose metabolism [128, 129]. These studies suggest that light regulates metabolism by influencing circadian rhythm. In addition to regulating metabolism by affecting circadian rhythm, Meng and colleagues found that acute light exposure decreases glucose tolerance in mice through a retina→hypothalamus→brown adipose tissue (BAT) pathway [130]. They demonstrated that ipRGCs directly innervate vasopressin neurons in the SON, which project to the PVN and then to the GABAergic neurons in the solitary tract nucleus, and eventually to BAT. Light-induced activation of this pathway blocks the adaptive thermogenesis in BAT, thereby decreasing glucose tolerance. Moreover, they found that acute light exposure (400 lx) during the daytime or nighttime also decreases glucose tolerance in humans, and these effects are probably involved in the inhibition of BAT-mediated adaptive thermogenesis. These results unveil a visual circuit linking the retina and BAT that mediates the effect of light on glucose metabolism. It would be interesting to test whether a light therapy paradigm targeting this visual circuit can alleviate the symptoms of metabolic disorders, such as obesity.
In addition to regulating metabolism through specific visual-related circuits, Richard A. Lang’s group found that light also regulates the adipose tissue-mediated adaptive thermogenesis through two non-visual-related circuits. In one study, they found that blue light directly activates encephalopsin (OPN3, a blue-light-responsive opsin) located in mouse adipocytes, which in turn increases thermogenesis [131]. In another study, they found that violet light (380 nm) directly activates the deep brain photosensitive protein OPN5 in the hypothalamic preoptic area, and then suppresses BAT thermogenesis [132]. These opposing activities of OPN3 and OPN5 on thermogenesis raise the hypothesis that non-visual-related circuits decode light information to help the organism to maintain homeostasis through calibrating BAT activity appropriate to the time of day.
Conclusions and Prospects
Growing evidence derived from both clinical trials and animal studies supports the beneficial effects of light therapy on multiple brain functions, including mood, cognition, sleep, and pain. However, the clinical application of light therapy has been faced with difficulties due to the unclear mechanism of action. As noted above, due to advances in research technologies, tremendous progress has been made in deciphering the circuit mechanisms of the effects of light on brain functions, such as mood, memory, pain perception, sleep, circadian rhythm, brain development, and metabolism. Several dedicated neural circuits have been demonstrated to mediate the beneficial and negative effects of light on brain functions. This experimental evidence not only provides a new theoretical basis for the application of light therapy in populations but also provides a new idea for developing novel light therapy strategies targeting specific visual circuits. Despite all these exciting advances, several key questions remain to be addressed.
Multiple key components of the neural circuit for non-visual light signal processing have been identified in the vLGN/IGL. However, an understanding of the vLGN/IGL-related circuits for light signal processing is not complete. For example, we found that vLGN/IGL neurons co-release GABA and glutamate and can be co-labeled by several traditional neural markers [91]. This suggests that vLGN/IGL neurons cannot be accurately classified by traditional neuron classification criteria. Fine-typing of vLGN/IGL neurons by single-cell transcriptome sequencing, thus revealing the neural connections and functions of different vLGN/IGL neural subtypes, is of great importance for further understanding the circuit mechanisms underlying the modulatory effects of light on brain functions.
Different light patterns can have opposite effects on the same brain function. For example, daytime bright light exposure may have antidepressant effects, while irregular light exposure may increase depression. It is important to establish new light therapy paradigms that can effectively activate specific visual circuits based on the unique light response properties of different visual circuits. This is crucial for the development of novel therapeutic strategies targeting the visual system to improve neurological diseases.
Finally, our knowledge of the effects of non-visual stimuli on the visual system is still limited and requires further investigation. For example, can changes in mood modulate the morphological and physiological properties of certain visual circuits? Can these effects impact the efficiency of light therapy? Systematic investigation of these questions will not only help to further understand the neural mechanisms of light therapy but also provide guidance for the development of new light therapy strategies.
References
Fu Y, Liao HW, Do MTH, Yau KW. Non-image-forming ocular photoreception in vertebrates. Curr Opin Neurobiol 2005, 15: 415–422.
LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 2014, 15: 443–454.
Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am J Psychiatry 2005, 162: 656–662.
Baron RA, Rea MS, Daniels SG. Effects of indoor lighting (illuminance and spectral distribution) on the performance of cognitive tasks and interpersonal behaviors: The potential mediating role of positive affect. Motiv Emot 1992, 16: 1–33.
Heschong L, Wright RL, Okura S. Daylighting impacts on human performance in school. J Illum Eng Soc 2002, 31: 101–114.
Mills PR, Tomkins SC, Schlangen LJM. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms 2007, 5: 2.
Viola AU, James LM, Schlangen LJM, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health 2008, 34: 297–306.
Barkmann C, Wessolowski N, Schulte-Markwort M. Applicability and efficacy of variable light in schools. Physiol Behav 2012, 105: 621–627.
Walch JM, Rabin BS, Day R, Williams JN, Choi K, Kang JD. The effect of sunlight on postoperative analgesic medication use: A prospective study of patients undergoing spinal surgery. Psychosom Med 2005, 67: 156–163.
Burgess HJ, Park M, Ong JC, Shakoor N, Williams DA, Burns J. Morning versus evening bright light treatment at home to improve function and pain sensitivity for women with fibromyalgia: A pilot study. Pain Med 2017, 18: 116–123.
Burgess HJ, Rizvydeen M, Kimura M, Pollack MH, Hobfoll SE, Rajan KB, et al. An open trial of morning bright light treatment among US military veterans with chronic low back pain: A pilot study. Pain Med 2019, 20: 770–778.
Shvarkov SB, Talitskaya OE, Neudakhin EV. Application of phototherapy in children with headache. Zhurnal Nevrologii I Psikhiatrii Imeni S S Korsakova 2000, 100: 40–42. Russian. PMID: 11195536.
Pjrek E, Friedrich ME, Cambioli L, Dold M, Jäger F, Komorowski A, et al. The efficacy of light therapy in the treatment of seasonal affective disorder: A meta-analysis of randomized controlled trials. Psychother Psychosom 2020, 89: 17–24.
Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: A randomized clinical trial. JAMA Psychiatry 2016, 73: 56–63.
Geoffroy PA, Schroder CM, Reynaud E, Bourgin P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med Rev 2019, 48: 101213.
Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002, 159: 666–669.
Jiang L, Zhang S, Wang Y, So KF, Ren C, Tao Q. Efficacy of light therapy for a college student sample with non-seasonal subthreshold depression: An RCT study. J Affect Disord 2020, 277: 443–449.
Pachito DV, Eckeli AL, Desouky AS, Corbett MA, Partonen T, Rajaratnam SM, et al. Workplace lighting for improving alertness and mood in daytime workers. Cochrane Database Syst Rev 2018, 3: CD012243.
Mu YM, Huang XD, Zhu S, Hu ZF, So KF, Ren CR, et al. Alerting effects of light in healthy individuals: A systematic review and meta-analysis. Neural Regen Res 2022, 17: 1929–1936.
Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci 2009, 13: 429–438.
Killgore WDS, Vanuk JR, Shane BR, Weber M, Bajaj S. A randomized, double-blind, placebo-controlled trial of blue wavelength light exposure on sleep and recovery of brain structure, function, and cognition following mild traumatic brain injury. Neurobiol Dis 2020, 134: 104679.
Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev 2014: CD003946.
Shishegar N, Boubekri M. Lighting up living spaces to improve mood and cognitive performance in older adults. J Environ Psychol 2022, 82: 101845.
Wu LM, Valdimarsdottir HB, Amidi A, Reid KJ, Ancoli-Israel S, Bovbjerg K, et al. Examining the efficacy of bright light therapy on cognitive function in hematopoietic stem cell transplant survivors. J Biol Rhythms 2022, 37: 471–483.
Kim SJ, Lee SH, Suh IB, Jang JW, Jhoo JH, Lee JH. Positive effect of timed blue-enriched white light on sleep and cognition in patients with mild and moderate Alzheimer’s disease. Sci Rep 2021, 11: 10174.
Hamilton M, Kwok WS, Hsu A, Mathieson S, Gnjidic D, Deyo R, et al. Opioid deprescribing in patients with chronic noncancer pain: A systematic review of international guidelines. Pain 2023, 164: 485–493.
Tan SY. Cognitive and cognitive-behavioral methods for pain control: A selective review. Pain 1982, 12: 201–228.
Neff KD. Self-compassion: Theory, method, research, and intervention. Annu Rev Psychol 2023, 74: 193–218.
Ma X, Chen W, Yang NN, Wang L, Hao XW, Tan CX, et al. Potential mechanisms of acupuncture for neuropathic pain based on somatosensory system. Front Neurosci 2022, 16: 940343.
Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol 2023, 19: 199–220.
Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010, 2010: CD006605.
Burns JW, Gerhart J, Rizvydeen M, Kimura M, Burgess HJ. Morning bright light treatment for chronic low back pain: Potential impact on the volatility of pain, mood, function, and sleep. Pain Med 2020, 21: 1153–1161.
Huang LB, Tsai MC, Chen CY, Hsu SC. The effectiveness of light/dark exposure to treat insomnia in female nurses undertaking shift work during the evening/night shift. J Clin Sleep Med 2013, 9: 641–646.
Lack L, Wright H, Paynter D. The treatment of sleep onset insomnia with bright morning light. Sleep Biol Rhythms 2007, 5: 173–179.
Lack L, Bramwell T, Wright H, Kemp K. Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms 2007, 5: 78–80.
Pallesen S, Nordhus IH, Skelton SH, Bjorvatn B, Skjerve A. Bright light treatment has limited effect in subjects over 55 years with mild early morning awakening. Percept Mot Skills 2005, 101: 759–770.
van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev 2016, 29: 52–62.
Cheng DCY, Ganner JL, Gordon CJ, Phillips CL, Grunstein RR, Comas M. The efficacy of combined bright light and melatonin therapies on sleep and circadian outcomes: A systematic review. Sleep Med Rev 2021, 58: 101491.
Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed light therapy for sleep and daytime sleepiness associated with parkinson disease: A randomized clinical trial. JAMA Neurol 2017, 74: 411–418.
Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci U S A 2006, 103: 7414–7419.
Burgess HJ, Fogg LF, Young MA, Eastman CI. Bright light therapy for winter depression—is phase advancing beneficial? Chronobiol Int 2004, 21: 759–775.
Hirakawa H, Terao T, Shirahama M. Light modulation for bipolar disorder: A commentary on “An update on adjunctive treatment options for bipolar disorder” by Dean et al. (2018). Bipolar Disord 2019, 21: 282.
Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, et al. Randomized clinical trial of bright light therapy for antepartum depression: Preliminary findings. J Clin Psychiatry 2004, 65: 421–425.
Wirz-Justice A, Bader A, Frisch U, Stieglitz RD, Alder J, Bitzer J, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry 2011, 72: 986–993.
Xiao M, Feng L, Wang Q, Luan X, Chen S, He J. The therapeutic effects and safety of bright light therapy combined with escitalopram oxalate on insomnia in patients with poststroke depression. Int J Geriatr Psychiatry 2021, 36: 182–189.
Perrin F, Peigneux P, Fuchs S, Verhaeghe S, Laureys S, Middleton B, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Curr Biol 2004, 14: 1842–1846.
Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol 2006, 16: 1616–1621.
Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex 2007, 17: 2788–2795.
Fisher PM, Madsen MK, Mc Mahon B, Holst KK, Andersen SB, Laursen HR, et al. Three-week bright-light intervention has dose-related effects on threat-related corticolimbic reactivity and functional coupling. Biol Psychiatry 2014, 76: 332–339.
Ma J, Kim M, Kim J, Hong G, Namgung E, Park S, et al. Decreased functional connectivity within the salience network after two-week morning bright light exposure in individuals with sleep disturbances: A preliminary randomized controlled trial. Sleep Med 2020, 74: 66–72.
Pickard GE, Sollars PJ. Intrinsically photosensitive retinal ganglion cells. Sci China Life Sci 2010, 53: 58–67.
Do MTH. Melanopsin and the intrinsically photosensitive retinal ganglion cells: Biophysics to behavior. Neuron 2019, 104: 205–226.
Sondereker KB, Stabio ME, Renna JM. Crosstalk: The diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J Comp Neurol 2020, 528: 2044–2067.
Lamb TD. Why rods and cones? Eye 2016, 30: 179–185.
Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295: 1070–1073.
Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295: 1065–1070.
Hannibal J, Fahrenkrug J. Melanopsin: A novel photopigment involved in the photoentrainment of the brain’s biological clock? Ann Med 2002, 34: 401–407.
Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol 2003, 460: 380–393.
Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002, 415: 493.
Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005, 433: 749–754.
Reifler AN, Chervenak AP, Dolikian ME, Benenati BA, Meyers BS, Demertzis ZD, et al. The rat retina has five types of ganglion-cell photoreceptors. Exp Eye Res 2015, 130: 17–28.
Hannibal J, Christiansen AT, Heegaard S, Fahrenkrug J, Kiilgaard JF. Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. J Comp Neurol 2017, 525: 1934–1961.
Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron 2010, 67: 49–60.
Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol 2010, 518: 2405–2422.
Li JY, Schmidt TM. Divergent projection patterns of M1 ipRGC subtypes. J Comp Neurol 2018, 526: 2010–2018.
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006, 497: 326–349.
Neumeister A, Turner EH, Matthews JR, Postolache TT, Barnett RL, Rauh M, et al. Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Arch Gen Psychiatry 1998, 55: 524–530.
Lam RW, Zis AP, Grewal A, Delgado PL, Charney DS, Krystal JH. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Arch Gen Psychiatry 1996, 53: 41–44.
Neumeister A, Praschak-Rieder N, Besselmann B, Rao ML, Glück J, Kasper S. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry 1997, 54: 133–138.
Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: Crossroad between the basal Ganglia and the limbic system. J Neurosci 2008, 28: 11825–11829.
Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience 2005, 132: 519–528.
Huang L, Xi Y, Peng Y, Yang Y, Huang X, Fu Y, et al. A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron 2019, 102: 128-142.e8.
Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, et al. Light affects mood and learning through distinct retina-brain pathways. Cell 2018, 175: 71-84.e18.
An K, Zhao H, Miao Y, Xu Q, Li YF, Ma YQ, et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat Neurosci 2020, 23: 869–880.
Canter RG, Penney J, Tsai LH. The Road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539: 187–196.
Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 2016, 17: 777–792.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540: 230–235.
Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DNW, et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 2019, 102: 929-943.e8.
Soula M, Martín-Ávila A, Zhang Y, Dhingra A, Nitzan N, Sadowski MJ, et al. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat Neurosci 2023, 26: 570–578.
Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci 2000, 54: 352–353.
Shan LL, Guo H, Song NN, Jia ZP, Hu XT, Huang JF, et al. Light exposure before learning improves memory consolidation at night. Sci Rep 2015, 5: 15578.
Warthen DM, Wiltgen BJ, Provencio I. Light enhances learned fear. Proc Natl Acad Sci U S A 2011, 108: 13788–13793.
Soler JE, Robison AJ, Núñez AA, Yan L. Light modulates hippocampal function and spatial learning in a diurnal rodent species: A study using male Nile grass rat (Arvicanthis niloticus). Hippocampus 2018, 28: 189–200.
Huang X, Huang P, Huang L, Hu Z, Liu X, Shen J, et al. A visual circuit related to the nucleus reuniens for the spatial-memory-promoting effects of light treatment. Neuron 2021, 109: 347-362.e7.
Klein MM, Cholvin T, Cosquer B, Salvadori A, Le Mero J, Kourouma L, et al. Ventral midline thalamus lesion prevents persistence of new (learning-triggered) hippocampal spines, delayed neocortical spinogenesis, and spatial memory durability. Brain Struct Funct 2019, 224: 1659–1676.
Jung D, Huh Y, Cho J. The ventral midline thalamus mediates hippocampal spatial information processes upon spatial cue changes. J Neurosci 2019, 39: 2276–2290.
Cholvin T, Hok V, Giorgi L, Chaillan FA, Poucet B. Ventral midline thalamus is necessary for hippocampal place field stability and cell firing modulation. J Neurosci 2018, 38: 158–172.
Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 2012, 27: 319–327.
Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain 2017, 158: 347–360.
Martin LF, Cheng K, Washington SM, Denton M, Goel V, Khandekar M, et al. Green light exposure elicits anti-inflammation, endogenous opioid release and dampens synaptic potentiation to relieve post-surgical pain. J Pain 2023, 24: 509–529.
Hu Z, Mu Y, Huang L, Hu Y, Chen Z, Yang Y, et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron 2022, 110: 1712-1727.e7.
Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 1995, 46: 575–605.
Behbehani MM, Jiang M, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain 1990, 40: 195–204.
Liebeskind JC, Guilbaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: Behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res 1973, 50: 441–446.
Samineni VK, Grajales-Reyes JG, Copits BA, O'Brien DE, Trigg SL, Gomez AM, et al. Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. eNeuro 2017, 4: ENEURO.0129–ENEURO.0116.2017.
Martin LF, Patwardhan AM, Jain SV, Salloum MM, Freeman J, Khanna R, et al. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia 2021, 41: 135–147.
Martin LF, Moutal A, Cheng K, Washington SM, Calligaro H, Goel V, et al. Green light antinociceptive and reversal of thermal and mechanical hypersensitivity effects rely on endogenous opioid system stimulation. J Pain 2021, 22: 1646–1656.
Martin L, Porreca F, Mata EI, Salloum M, Goel V, Gunnala P, et al. Green light exposure improves pain and quality of life in fibromyalgia patients: A preliminary one-way crossover clinical trial. Pain Med 2021, 22: 118–130.
Tang YL, Liu AL, Lv SS, Zhou ZR, Cao H, Weng SJ, et al. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci Transl Med 2022, 14: eabq6474.
Cao P, Zhang M, Ni Z, Song XJ, Yang CL, Mao Y, et al. Green light induces antinociception via visual-somatosensory circuits. Cell Rep 2023, 42: 112290.
Mistlberger RE. Circadian regulation of sleep in mammals: Role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 2005, 49: 429–454.
Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol 2010, 72: 551–577.
Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One 2008, 3: e2451.
Chew KS, Renna JM, McNeill DS, Fernandez DC, Keenan WT, Thomsen MB, et al. A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. Elife 2017, 6: e22861.
Göz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS One 2008, 3: e3153.
Kofuji P, Mure LS, Massman LJ, Purrier N, Panda S, Engeland WC. Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS One 2016, 11: e0168651.
Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Xie X, et al. Role of melanopsin in circadian responses to light. Science 2002, 298: 2211–2213.
Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298: 2213–2216.
Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424: 76–81.
Rupp AC, Ren M, Altimus CM, Fernandez DC, Richardson M, Turek F, et al. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. Elife 2019, 8: e44358.
Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci 2008, 11: 1068–1073.
Zhang Z, Liu WY, Diao YP, Xu W, Zhong YH, Zhang JY, et al. Superior Colliculus GABAergic neurons are essential for acute dark induction of wakefulness in mice. Curr Biol 2019, 29: 637-644.e3.
Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron 2005, 48: 465–477.
Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science 1993, 260: 1819–1821.
Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, Yao H, et al. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci 2014, 17: 391–399.
Tarttelin EE, Bellingham J, Bibb LC, Foster RG, Hankins MW, Gregory-Evans K, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res 2003, 76: 393–396.
Hu J, Shi Y, Zhang J, Huang X, Wang Q, Zhao H, et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell 2022, 185: 3124-3137.e15.
Fernandez DC, Komal R, Langel J, Ma J, Duy PQ, Penzo MA, et al. Retinal innervation tunes circuits that drive nonphotic entrainment to food. Nature 2020, 581: 194–198.
van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord 1999, 23: 973–978.
Parkes KR. Shift work and age as interactive predictors of body mass index among offshore workers. Scand J Work Environ Health 2002, 28: 64–71.
Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health 2003, 76: 424–430.
Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health 2005, 47: 89–95.
Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 2019, 20: 227–241.
Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol 2019, 15: 75–89.
Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 2010, 107: 18664–18669.
Bedrosian TA, Galan A, Vaughn CA, Weil ZM, Nelson RJ. Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters. J Neuroendocrinol 2013, 25: 590–596.
Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009, 106: 4453–4458.
Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev 2014, 35: 648–670.
Russart KLG, Nelson RJ. Light at night as an environmental endocrine disruptor. Physiol Behav 2018, 190: 82–89.
Meng JJ, Shen JW, Li G, Ouyang CJ, Hu JX, Li ZS, et al. Light modulates glucose metabolism by a retina-hypothalamus-brown adipose tissue axis. Cell 2023, 186: 398-412.e17.
Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, et al. Adaptive thermogenesis in mice is enhanced by opsin 3-dependent adipocyte light sensing. Cell Rep 2020, 30: 672-686.e8.
Zhang KX, D’Souza S, Upton BA, Kernodle S, Vemaraju S, Nayak G, et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature 2020, 585: 420–425.
Acknowledgements
This review was supported by grants from the National Natural Science Foundation of China (32171010 and 32100820), STI2030-Major Projects (2021ZD0203100), and the Guangdong Basic and Applied Basic Research Foundation (2023B1515040010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, X., Tao, Q. & Ren, C. A Comprehensive Overview of the Neural Mechanisms of Light Therapy. Neurosci. Bull. 40, 350–362 (2024). https://doi.org/10.1007/s12264-023-01089-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-023-01089-8