Abstract
Major depressive disorder (MDD), also referred to as depression, is one of the most common psychiatric disorders with a high economic burden. The etiology of depression is still not clear, but it is generally believed that MDD is a multifactorial disease caused by the interaction of social, psychological, and biological aspects. Therefore, there is no exact pathological theory that can independently explain its pathogenesis, involving genetics, neurobiology, and neuroimaging. At present, there are many treatment measures for patients with depression, including drug therapy, psychotherapy, and neuromodulation technology. In recent years, great progress has been made in the development of new antidepressants, some of which have been applied in the clinic. This article mainly reviews the research progress, pathogenesis, and treatment of MDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Major depressive disorder (MDD) also referred to as depression, is one of the most severe and common psychiatric disorders across the world. It is characterized by persistent sadness, loss of interest or pleasure, low energy, worse appetite and sleep, and even suicide, disrupting daily activities and psychosocial functions. Depression has an extreme global economic burden and has been listed as the third largest cause of disease burden by the World Health Organization since 2008, and is expected to rank the first by 2030 [1, 2]. In 2016, the Global Burden of Diseases, Injuries, and Risk Factors Study demonstrated that depression caused 34.1 million of the total years lived with disability (YLDs), ranking as the fifth largest cause of YLD [3]. Therefore, the research progress and the clinical application of new discoveries or new technologies are imminent. In this review, we mainly discuss the current situation of research, developments in pathogenesis, and the management of depression.
Current Situation of Research on Depression
Analysis of Published Papers
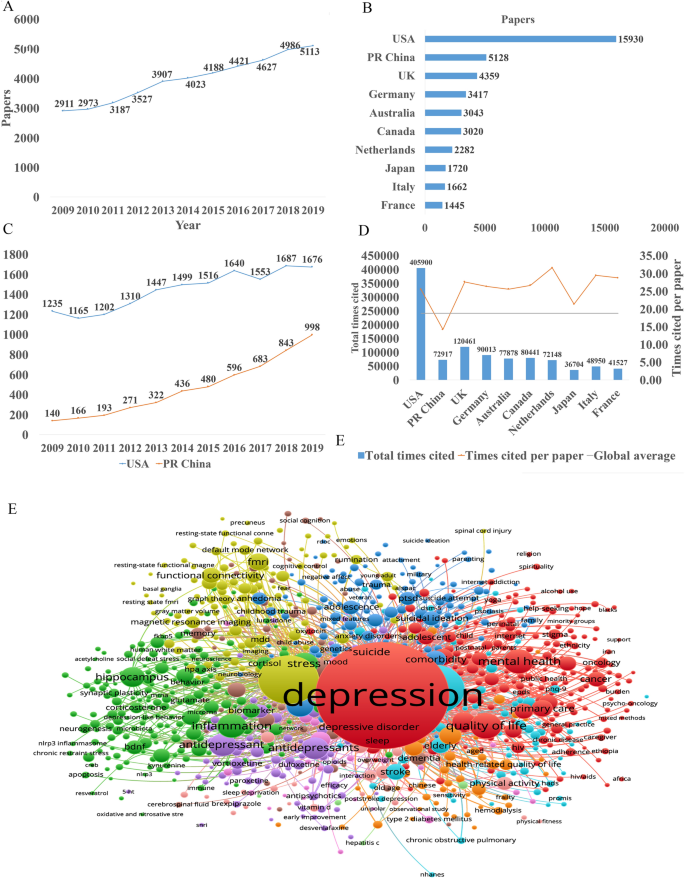
In the past decade, the total number of papers on depression published worldwide has increased year by year as shown in Fig. 1A. Searching the Web of Science database, we found a total of 43,863 papers published in the field of depression from 2009 to 2019 (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009–2019), Articles). The top 10 countries that published papers on the topic of depression are shown in Fig. 1B. Among them, researchers in the USA published the most papers, followed by China. Compared with the USA, the gap in the total number of papers published in China is gradually narrowing (Fig. 1C), but the quality gap reflected by the index (the total number of citations and the number of citations per paper) is still large, and is lower than the global average (Fig. 1D). As shown in Fig. 1E, the hot research topics in depression are as follows: depression management in primary care, interventions to prevent depression, the pathogenesis of depression, comorbidity of depression and other diseases, the risks of depression, neuroimaging studies of depression, and antidepressant treatment.
Analysis of published papers around the world from 2009 to 2019 in depressive disorder. A The total number of papers [from a search of the Web of Science database (search strategy: TI = (depression$) or ts = ("major depressive disorder$")) and py = (2009–2019), Articles)]. B The top 10 countries publishing on the topic. C Comparison of papers in China and the USA. D Citations for the top 10 countries and comparison with the global average. E Hot topics.
Analysis of Patented Technology Application
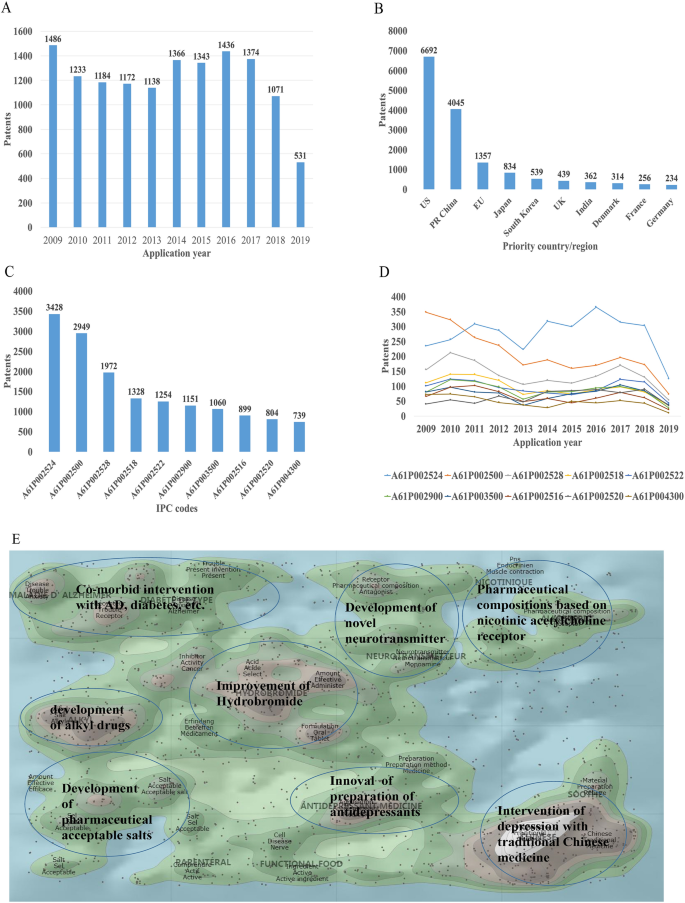
There were 16,228 patent applications in the field of depression between 2009 and 2019, according to the Derwent Innovation Patent database. The annual number and trend of these patents are shown in Fig. 2A. The top 10 countries applying for patents related to depression are shown in Fig. 2B. The USA ranks first in the number of depression-related patent applications, followed by China. The largest number of patents related to depression is the development of antidepressants, and drugs for neurodegenerative diseases such as dementia comorbid with depression. The top 10 technological areas of patents related to depression are shown in Fig. 2C, and the trend in these areas have been stable over the past decade (Fig. 2D).
Analysis of patented technology applications from 2009 to 2019 in the field of depressive disorder. A Annual numbers and trends of patents (the Derwent Innovation patent database). B The top 10 countries/regions applying for patents. C The top 10 technological areas of patents. D The trend of patent assignees. E Global hot topic areas of patents.
Analysis of technical hotspots based on keyword clustering was conducted from the Derwent Innovation database using the "ThemeScape" tool. This demonstrated that the hot topic areas are as follows (Fig. 2E): (1) improvement for formulation and the efficiency of hydrobromide, as well as optimization of the dosage; intervention for depression comorbid with AD, diabetes, and others; (3) development of alkyl drugs; (4) development of pharmaceutical acceptable salts as antidepressants; (5) innovation of the preparation of antidepressants; (6) development of novel antidepressants based on neurotransmitters; (7) development of compositions based on nicotinic acetylcholine receptors; and (8) intervention for depression with traditional Chinese medicine.
Analysis of Clinical Trial
There are 6,516 clinical trials in the field of depression in the ClinicalTrials.gov database, and among them, 1,737 valid trials include the ongoing recruitment of subjects, upcoming recruitment of subjects, and ongoing clinical trials. These clinical trials are mainly distributed in the USA (802 trials), Canada (155), China (114), France (93), Germany (66), UK (62), Spain (58), Denmark (41), Sweden (39), and Switzerland (23). The indications for clinical trials include various types of depression, such as minor depression, depression, severe depression, perinatal depression, postpartum depression, and depression comorbid with other psychiatric disorders or physical diseases, such as schizophrenia, epilepsy, stroke, cancer, diabetes, cardiovascular disease, and Parkinson's disease.
Based on the database of the Chinese Clinical Trial Registry website, a total of 143 clinical trials for depression have been carried out in China. According to the type of research, they are mainly interventional and observational studies, as well as a small number of related factor studies, epidemiological studies, and diagnostic trials. The research content involves postpartum, perinatal, senile, and other age groups with clinical diagnosis (imaging diagnosis) and intervention studies (drugs, acupuncture, electrical stimulation, transcranial magnetic stimulation). It also includes intervention studies on depression comorbid with coronary heart disease, diabetes, and heart failure.
New Medicine Development
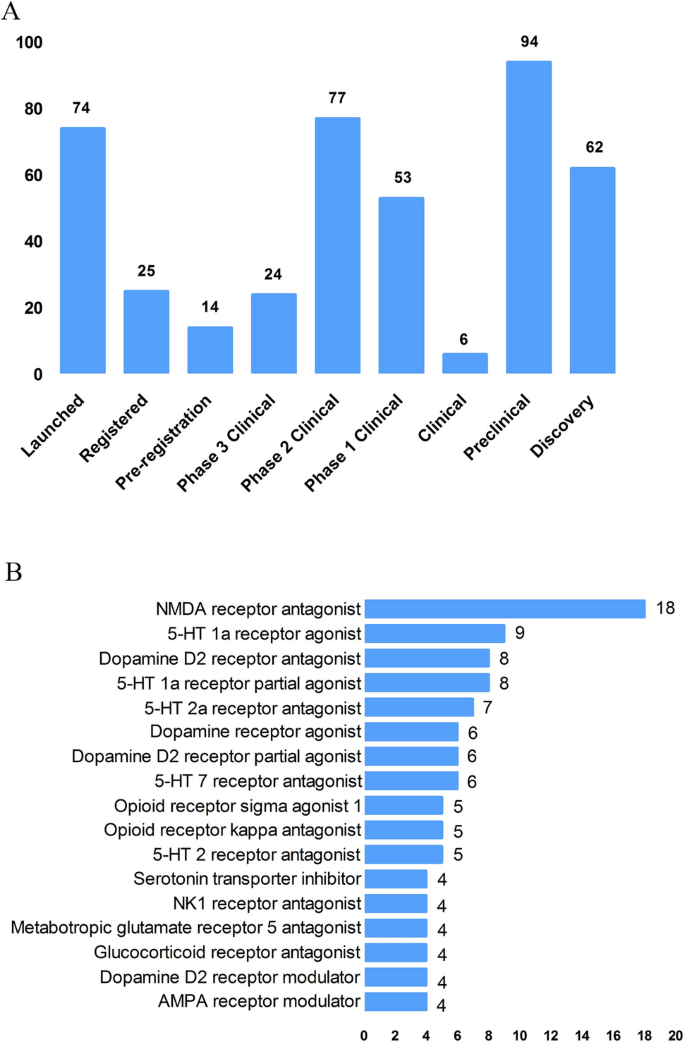
According to the Cortellis database, 828 antidepressants were under development by the end of 2019, but only 292 of these are effective and active (Fig. 3A). Large number of them have been discontinued or made no progress, indicating that the development of new drugs in the field of depression is extremely urgent.
From the perspective of target-based actions, the most common new drugs are NMDA receptor antagonists, followed by 5-HT targets, as well as dopamine receptor agonists, opioid receptor antagonists and agonists, AMPA receptor modulators, glucocorticoid receptor antagonists, NK1 receptor antagonists, and serotonin transporter inhibitors (Fig. 3B).
Epidemiology of Depression
The prevalence of depression varies greatly across cultures and countries. Previous surveys have demonstrated that the 12-month prevalence of depression was 0.3% in the Czech Republic, 10% in the USA, 4.5% in Mexico, and 5.2% in West Germany, and the lifetime prevalence of depression was 1.0% in the Czech Republic, 16.9% in the USA, 8.3% in Canada, and 9.0% in Chile [4, 5]. A recent meta-analysis including 30 Countries showed that lifetime and 12-month prevalence depression were 10.8% and 7.2%, respectively [6]. In China, the lifetime prevalence of depression ranged from 1.6% to 5.5% [7,8,9]. An epidemiological study demonstrated that depression was the most common mood disorder with a life prevalence of 3.4% and a 12-month prevalence of 2.1% in China [10].
Some studies have also reported the prevalence in specific populations. The National Comorbidity Survey-Adolescent Supplement (NCS-A) survey in the USA showed that the lifetime and 12-month prevalence of depression in adolescents aged 13 to 18 were 11.0% and 7.5%, respectively [11]. A recent meta-analysis demonstrated that lifetime prevalence and 12-month prevalence were 2.8% and 2.3%, respectively, among the elderly population in China [12].
Neurobiological Pathogenesis of Depressive Disorder
Monoamines
The early hypothesis of monoamines in the pathophysiology of depression has been accepted by the scientific community. The evidence that monoamine oxidase inhibitors and tricyclic antidepressants promote monoamine neurotransmission supports this theory of depression [13]. So far, selective serotonin reuptake inhibitors and norepinephrine reuptake inhibitors are still the first-line antidepressants. However, there remain 1/3 to 2/3 of depressed patients who do not respond satisfactorily to initial antidepressant treatment, and even as many as 15%–40% do not respond to several pharmacological medicines [14, 15]. Therefore, the underlying pathogenesis of depression is far beyond the simple monoamine mechanism.
Other hypotheses of depression have gradually received increasing attention because of biomarkers for depression and the effects pharmacological treatments, such as the stress-responsive hypothalamic pituitary adrenal (HPA) axis, neuroendocrine systems, the neurotrophic family of growth factors, and neuroinflammation.
Stress-Responsive HPA Axis
Stress is causative or a contributing factor to depression. Particularly, long-term or chronic stress can lead to dysfunction of the HPA axis and promote the secretion of hormones, including cortisol, adrenocorticotropic hormone, corticotropin-releasing hormone, arginine vasopressin, and vasopressin. About 40%–60% of patients with depression display a disturbed HPA axis, including hypercortisolemia, decreased rhythmicity, and elevated cortisol levels [16, 17]. Mounting evidence has shown that stress-induced abnormality of the HPA axis is associated with depression and cognitive impairment, which is due to the increased secretion of cortisol and the insufficient inhibition of glucocorticoid receptor regulatory feedback [18, 19]. In addition, it has been reported that the increase in cortisol levels is related to the severity of depression, especially in melancholic depression [20, 21]. Further, patients with depression whose HPA axis was not normalized after treatment had a worse clinical response and prognosis [22, 23]. Despite the above promising insights, unfortunately previous studies have shown that treatments regulating the HPA axis, such as glucocorticoid receptor antagonists, do not attenuate the symptoms of depressed patients [24, 25].
Glutamate Signaling Pathway
Glutamate is the main excitatory neurotransmitter released by synapses in the brain; it is involved in synaptic plasticity, cognitive processes, and reward and emotional processes. Stress can induce presynaptic glutamate secretion by neurons and glutamate strongly binds to ionotropic glutamate receptors (iGluRs) including N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) [26] on the postsynaptic membrane to activate downstream signal pathways [27]. Accumulating evidence has suggested that the glutamate system is associated with the incidence of depression. Early studies have shown increased levels of glutamate in the peripheral blood, cerebrospinal fluid, and brain of depressed patients [28, 29], as well as NMDAR subunit disturbance in the brain [30, 31]. Blocking the function of NMDARs has an antidepressant effect and protects hippocampal neurons from morphological abnormalities induced by stress, while antidepressants reduce glutamate secretion and NMDARs [32]. Most importantly, NMDAR antagonists such as ketamine have been reported to have profound and rapid antidepressant effects on both animal models and the core symptoms of depressive patients [33]. On the other hand, ketamine can also increase the AMPAR pathway in hippocampal neurons by up-regulating the AMPA glutamate receptor 1 subunit [34]. Further, the AMPAR pathway may be involved in the mechanism of antidepressant effects. For example, preclinical studies have indicated that AMPAR antagonists might attenuate lithium-induced depressive behavior by increasing the levels of glutamate receptors 1 and 2 in the mouse hippocampus [35].
Gamma-Aminobutyric Acid (GABA)
Contrary to glutamate, GABA is the main inhibitory neurotransmitter. Although GABA neurons account for only a small proportion compared to glutamate, inhibitory neurotransmission is essential for brain function by balancing excitatory transmission [36]. Number of studies have shown that patients with depression have neurotransmission or functional defects of GABA [37, 38]. Schür et al., conducted a meta-analysis of magnetic resonance spectroscopy studies, which showed that the brain GABA level in depressive patients was lower than that in healthy controls, but no difference was found in depressive patients in remission [39]. Several postmortem studies have shown decreased levels of the GABA synthase glutamic acid decarboxylase in the prefrontal cortex of patients with depression [40, 41]. It has been suggested that a functional imbalance of the GABA and glutamate systems contributes to the pathophysiology of depression, and activation of the GABA system might induce antidepressant activity, by which GABAA receptor mediators α2/α3 are considered potential antidepressant candidates [42, 43]. Genetic mouse models, such as the GABAA receptor mutant mouse and conditional the Gad1-knockout mouse (GABA in hippocampus and cerebral cortex decreased by 50%) and optogenetic methods have verified that depression-like behavior is induced by changing the level of GABA [44, 45].
Neurotrophin Family
The neurotrophin family plays a key role in neuroplasticity and neurogenesis. The neurotrophic hypothesis of depression postulates that a deficit of neurotrophic support leads to neuronal atrophy, the reduction of neurogenesis, and the destruction of glia support, while antidepressants attenuate or reverse these pathophysiological processes [46]. Among them, the most widely accepted hypothesis involves brain-derived neurotrophic factor (BDNF). This was initially triggered by evidence that stress reduces the BDNF levels in the animal brain, while antidepressants rescue or attenuate this reduction [47, 48], and agents involved in the BDNF system have been reported to exert antidepressant-like effects [49, 50]. In addition, mounting studies have reported that the BDNF level is decreased in the peripheral blood and at post-mortem in depressive patients, and some have reported that antidepressant treatment normalizes it [51, 52]. Furthermore, some evidence also showed that the interaction of BDNF and its receptor gene is associated with treatment-resistant depression [15].
Recent studies reported that depressed patients have a lower level of the pro-domain of BDNF (BDNF pro-peptide) than controls. This is located presynaptically and promotes long-term depression in the hippocampus, suggesting that it is a promising synaptic regulator [53].
Neuroinflammation
The immune-inflammation hypothesis has attracted much attention, suggesting that the interactions between inflammatory pathways and neural circuits and neurotransmitters are involved in the pathogenesis and pathophysiological processes of depression. Early evidence found that patients with autoimmune or infectious diseases are more likely to develop depression than the general population [54]. In addition, individuals without depression may display depressive symptoms after treatment with cytokines or cytokine inducers, while antidepressants relieve these symptoms [55, 56]. There is a complex interaction between the peripheral and central immune systems. Previous evidence suggested that peripheral inflammation/infection may spread to the central nervous system in some way and cause a neuroimmune response [55, 57]: (1) Some cytokines produced in the peripheral immune response, such as IL-6 and IL-1 β, can leak into the brain through the blood-brain barrier (BBB). (2) Cytokines entering the central nervous system act directly on astrocytes, small stromal cells, and neurons. (3) Some peripheral immune cells can cross the BBB through specific transporters, such as monocytes. (4) Cytokines and chemokines in the circulation activate the central nervous system by regulating the surface receptors of astrocytes and endothelial cells at the BBB. (5) As an intermediary pathway, the immune inflammatory response transmits peripheral danger signals to the center, amplifies the signals, and shows the external phenotype of depressive behavior associated with stress/trauma/infection. (6) Cytokines and chemokines may act directly on neurons, change their plasticity and promote depression-like behavior.
Patients with depression show the core feature of the immune-inflammatory response, that is, increased concentrations of pro-inflammatory cytokines and their receptors, chemokines, and soluble adhesion molecules in peripheral blood and cerebrospinal fluid [58,59,60]. Peripheral immune-inflammatory response markers not only change the immune activation state in the brain that affects explicit behavior, but also can be used as an evaluation index or biological index of antidepressant therapy [61, 62]. Li et al. showed that the level of TNF-α in patients with depression prior to treatment was higher than that in healthy controls. After treatment with venlafaxine, the level of TNF-α in patients with depression decreased significantly, and the level of TNF-α in the effective group decreased more [63]. A recent meta-analysis of 1,517 patients found that antidepressants significantly reduced peripheral IL-6, TNF-α, IL-10, and CCL-2, suggesting that antidepressants reduce markers of peripheral inflammatory factors [64]. Recently, Syed et al. also confirmed that untreated patients with depression had higher levels of inflammatory markers and increased levels of anti-inflammatory cytokines after antidepressant treatment, while increased levels of pro-inflammatory cytokines were found in non-responders [62]. Clinical studies have also found that anti-inflammatory cytokines, such as monoclonal antibodies and other cytokine inhibitors, may play an antidepressant role by blocking cytokines. The imbalance of pro-inflammatory and anti-inflammatory cytokines may be involved in the pathophysiological process of depression.
In addition, a recent study showed that microglia contribute to neuronal plasticity and neuroimmune interaction that are involved in the pathophysiology of depression [65]. When activated microglia promote inflammation, especially the excessive production of pro-inflammatory factors and cytotoxins in the central nervous system, depression-like behavior can gradually develop [65, 66]. However, microglia change polarization as two types under different inflammatory states, regulating the balance of pro- and anti-inflammatory factors. These two types are M1 and M2 microglia; the former produces large number of pro-inflammatory cytokines after activation, and the latter produces anti-inflammatory cytokines. An imbalance of M1/M2 polarization of microglia may contribute to the pathophysiology of depression [67].
Microbiome-Gut-Brain Axis
The microbiota-gut-brain axis has recently gained more attention because of its ability to regulate brain activity. Many studies have shown that the microbiota-gut-brain axis plays an important role in regulating mood, behavior, and neuronal transmission in the brain [68, 69]. It is well established that comorbidity of depression and gastrointestinal diseases is common [70, 71]. Some antidepressants can attenuate the symptoms of patients with irritable bowel syndrome and eating disorders [72]. It has been reported that gut microbiome alterations are associated with depressive-like behaviors [73, 74], and brain function [75]. Early animal studies have shown that stress can lead to long-term changes in the diversity and composition of intestinal microflora, and is accompanied by depressive behavior [76, 77]. Interestingly, some evidence indicates that rodents exhibit depressive behavior after fecal transplants from patients with depression [74]. On the other hand, some probiotics attenuated depressive-like behavior in animal studies, [78] and had antidepressant effects on patients with depression in several double-blind, placebo-controlled clinical trials [79, 80].
The potential mechanism may be that gut microbiota can interact with the brain through a variety of pathways or systems, including the HPA axis, and the neuroendocrine, autonomic, and neuroimmune systems [81]. For example, recent evidence demonstrated that gut microbiota can affect the levels of neurotransmitters in the gut and brain, including serotonin, dopamine, noradrenalin, glutamate, and GABA [82]. In addition, recent studies showed that changes in gut microbiota can also impair the gut barrier and promote higher levels of peripheral inflammatory cytokines [83, 84]. Although recent research in this area has made significant progress, more clinical trials are needed to determine whether probiotics have any effect on the treatment of depression and what the potential underlying mechanisms are.
Other Systems and Pathways
There is no doubt that several other systems or pathways are also involved in the pathophysiology of depression, such as oxidant-antioxidant imbalance [85], mitochondrial dysfunction [86, 87], and circadian rhythm-related genes [88], especially their critical interactions (e.g. interaction between the HPA and mitochondrial metabolism [89, 90], and the reciprocal interaction between oxidative stress and inflammation [2, 85]). The pathogenesis of depression is complex and all the hypotheses should be integrated to consider the many interactions between various systems and pathways.
Advances in Various Kinds of Research on Depressive Disorder
Genetic, molecular, and neuroimaging studies continue to increase our understanding of the neurobiological basis of depression. However, it is still not clear to what extent the results of neurobiological studies can help improve the clinical and functional prognosis of patients. Therefore, over the past 10 years, the neurobiological study of depression has become an important measure to understand the pathophysiological mechanism and guide the treatment of depression.
Genetic Studies
Previous twin and adoption studies have indicated that depression has relatively low rate of heritability at 37% [91]. In addition, environmental factors such as stressful events are also involved in the pathogenesis of depression. Furthermore, complex psychiatric disorders, especially depression, are considered to be polygenic effects that interact with environmental factors [13]. Therefore, reliable identification of single causative genes for depression has proved to be challenging. The first genome-wide association studies (GWAS) for depression was published in 2009, and included 1,738 patients and 1,802 controls [92, 93]. Although many subsequent GWASs have determined susceptible genes in the past decade, the impact of individual genes is so small that few results can be replicated [94, 95]. So far, it is widely accepted that specific single genetic mutations may play minor and marginal roles in complex polygenic depression. Another major recognition in GWASs over the past decade is that prevalent candidate genes are usually not associated with depression. Further, the inconsistent results may also be due to the heterogeneity and polygenic nature of genetic and non-genetic risk factors for depression as well as the heterogeneity of depression subtypes [95, 96]. Therefore, to date, the quality of research has been improved in two aspects: (1) the sample size has been maximized by combining the data of different evaluation models; and (2) more homogenous subtypes of depression have been selected to reduce phenotypic heterogeneity [97]. Levinson et al. pointed out that more than 75,000 to 100,000 cases should be considered to detect multiple depression associations [95]. Subsequently, several recent GWASs with larger sample sizes have been conducted. For example, Okbay et al. identified two loci associated with depression and replicated them in separate depression samples [98]. Wray et al. also found 44 risk loci associated with depression based on 135,458 cases and 344,901 controls [99]. A recent GWAS of 807,553 individuals with depression reported that 102 independent variants were associated with depression; these were involved in synaptic structure and neural transmission, and were verified in a further 1,507,153 individuals [100]. However, even with enough samples, GWASs still face severe challenges. A GWAS only marks the region of the genome and is not directly related to the potential biological function. In addition, a genetic association with the indicative phenotype of depression may only be part of many pathogenic pathways, or due to the indirect influence of intermediate traits in the causal pathway on the final result [101].
Given the diversity of findings, epigenetic factors are now being investigated. Recent studies indicated that epigenetic mechanisms may be the potential causes of "loss of heritability" in GWASs of depression. Over the past decade, a promising discovery has been that the effects of genetic information can be directly influenced by environment factors, and several specific genes are activated by environmental aspects. This process is described as interactions between genes and the environment, which is identified by the epigenetic mechanism. Environmental stressors cause alterations in gene expression in the brain, which may cause abnormal neuronal plasticity in areas related to the pathogenesis of the disease. Epigenetic events alter the structure of chromatin, thereby regulating gene expression involved in neuronal plasticity, stress behavior, depressive behavior, and antidepressant responses, including DNA methylation, histone acetylation, and the role of non-coding RNA. These new mechanisms of trans-generational transmission of epigenetic markers are considered a supplement to orthodox genetic heredity, providing the possibility for the discovery of new treatments for depression [102, 103]. Recent studies imply that life experiences, including stress and enrichment, may affect cellular and molecular signaling pathways in sperm and influence the behavioral and physiological phenotypes of offspring in gender-specific patterns, which may also play an important role in the development of depression [103].
Brain Imaging and Neuroimaging Studies
Neuroimaging, including magnetic resonance imaging (MRI) and molecular imaging, provides a non-invasive technique for determining the underlying etiology and individualized treatment for depression. MRI can provide important data on brain structure, function, networks, and metabolism in patients with depression; it includes structural MRI (sMRI), functional MRI (fMRI), diffusion tensor imaging, and magnetic resonance spectroscopy.
Previous sMRI studies have found damaged gray matter in depression-associated brain areas, including the frontal lobe, anterior cingulate gyrus, hippocampus, putamen, thalamus, and amygdala. sMRI focuses on the thickness of gray matter and brain morphology [104, 105]. A recent meta-analysis of 2,702 elderly patients with depression and 11,165 controls demonstrated that the volumes of the whole brain and hippocampus of patients with depression were lower than those of the control group [106]. Some evidence also showed that the hippocampal volume in depressive patients was lower than that of controls, and increased after treatment with antidepressants [107] and electroconvulsive therapy (ECT) [108], suggesting that the hippocampal volume plays a critical role in the development, treatment response, and clinical prognosis of depression. A recent study also reported that ECT increased the volume of the right hippocampus, amygdala, and putamen in patients with treatment-resistant depression [109]. In addition, postmortem research supported the MRI study showing that dentate gyrus volume was decreased in drug-naive patients with depression compared to healthy controls, and was potentially reversed by treatment with antidepressants [110].
Diffusion tensor imaging detects the microstructure of the white matter, which has been reported impaired in patients with depression [111]. A recent meta-analysis that included first-episode and drug-naïve depressive patients showed that the decrease in fractional anisotropy was negatively associated with illness duration and clinical severity [112].
fMRI, including resting-state and task-based fMRI, can divide the brain into self-related regions, such as the anterior cingulate cortex, posterior cingulate cortex, medial prefrontal cortex, precuneus, and dorsomedial thalamus. Many previous studies have shown the disturbance of several brain areas and intrinsic neural networks in patients with depression which could be rescued by antidepressants [113,114,115,116]. Further, some evidence also showed an association between brain network dysfunction and the clinical correlates of patients with depression, including clinical symptoms [117] and the response to antidepressants [118, 119], ECT [120, 121], and repetitive transcranial magnetic stimulation [122].
It is worth noting that brain imaging provides new insights into the large-scale brain circuits that underlie the pathophysiology of depressive disorder. In such studies, large-scale circuits are often referred to as “networks”. There is evidence that a variety of circuits are involved in the mechanisms of depressive disorder, including disruption of the default mode, salience, affective, reward, attention, and cognitive control circuits [123]. Over the past decade, the study of intra-circuit and inter-circuit connectivity dysfunctions in depression has escalated, in part due to advances in precision imaging and analysis techniques [124]. Circuit dysfunction is a potential biomarker to guide psychopharmacological treatment. For example, Williams et al. found that hyper-activation of the amygdala is associated with a negative phenotype that can predict the response to antidepressants [125]. Hou et al. showed that the baseline characteristics of the reward circuit predict early antidepressant responses [126].
Molecular imaging studies, including single photon emission computed tomography and positron emission tomography, focus on metabolic aspects such as amino-acids, neurotransmitters, glucose, and lipids at the cellular level in patients with depression. A recent meta-analysis examined glucose metabolism and found that glucose uptake dysfunction in different brain regions predicts the treatment response [127].
The most important and promising studies were conducted by the ENIGMA (Enhancing NeuroImaging Genetics through Meta Analysis) Consortium, which investigated the human brain across 43 countries. The ENIGMA-MDD Working Group was launched in 2012 to detect the structural and functional changes associated with MDD reliably and replicate them in various samples around the world [128]. So far, the ENIGMA-MDD Working Group has collected data from 4,372 MDD patients and 9,788 healthy controls across 14 countries, including 45 cohorts [128]. Their findings to date are shown in Table 1 [128,129,130,131,132,133,134,135,136,137].
Objective Index for Diagnosis of MDD
To date, the clinical diagnosis of depression is subjectively based on interviews according to diagnostic criteria (e.g. International Classification of Diseases and Diagnostic and Statistical Manual diagnostic systems) and the severity of clinical symptoms are assessed by questionnaires, although patients may experience considerable differences in symptoms and subtypes [138]. Meanwhile, biomarkers including genetics, epigenetics, peripheral gene and protein expression, and neuroimaging markers may provide a promising supplement for the development of the objective diagnosis of MDD, [139,140,141]. However, the development of reliable diagnosis for MDD using biomarkers is still difficult and elusive, and all methods based on a single marker are insufficiently specific and sensitive for clinical use [142]. Papakostas et al. showed that a multi-assay, serum-based test including nine peripheral biomarkers (soluble tumor necrosis factor alpha receptor type II, resistin, prolactin, myeloperoxidase, epidermal growth factor, BDNF, alpha1 antitrypsin, apolipoprotein CIII, brain-derived neurotrophic factor, and cortisol) yielded a specificity of 81.3% and a sensitivity of 91.7% [142]. However, the sample size was relatively small and no other studies have yet validated their results. Therefore, further studies are needed to identify biomarker models that integrate all biological variables and clinical features to improve the specificity and sensitivity of diagnosis for MDD.
Management of Depression
The treatment strategies for depression consist of pharmacological treatment and non-pharmacological treatments including psychotherapy, ECT [98], and transcranial magnetic stimulation. As psychotherapy has been shown to have effects on depression including attenuating depressive symptoms and improving the quality of life [143, 144]; several practice guidelines are increasingly recommending psychotherapy as a monotherapy or in combination with antidepressants [145, 146].
Current Antidepressant Treatment
Antidepressants approved by the US Food and Drug Administration (FDA) are shown in Table 2. Due to the relatively limited understanding of the etiology and pathophysiology of depression, almost all the previous antidepressants were discovered by accident a few decades ago. Although most antidepressants are usually safe and effective, there are still some limitations, including delayed efficacy (usually 2 weeks) and side-effects that affect the treatment compliance [147]. In addition, <50% of all patients with depression show complete remission through optimized treatment, including trials of multiple drugs with and without simultaneous psychotherapy. In the past few decades, most antidepressant discoveries focused on finding faster, safer, and more selective serotonin or norepinephrine receptor targets. In addition, there is an urgent need to develop new approaches to obtain more effective, safer, and faster antidepressants. In 2019, the FDA approved two new antidepressants: Esketamine for refractory depression and Bresanolone for postpartum depression. Esmolamine, a derivative of the anesthetic drug ketamine, was approved by the FDA for the treatment of refractory depression, based on a large number of preliminary clinical studies [148]. For example, several randomized controlled trials and meta-analysis studies showed the efficacy and safety of Esketamine in depression or treatment-resistant depression [26, 149, 150]. Although both are groundbreaking new interventions for these debilitating diseases and both are approved for use only under medical supervision, there are still concerns about potential misuse and problems in the evaluation of mental disorders [151].
To date, although several potential drugs have not yet been approved by the FDA, they are key milestones in the development of antidepressants that may be modified and used clinically in the future, such as compounds containing dextromethorphan (a non-selective NMDAR antago–nist), sarcosine (N-methylglycine, a glycine reuptake inhibitor), AMPAR modulators, and mGluR modulators [152].
Neuromodulation Therapy
Neuromodulation therapy acts through magnetic pulse, micro-current, or neural feedback technology within the treatment dose, acting on the central or peripheral nervous system to regulate the excitatory/inhibitory activity to reduce or attenuate the symptoms of the disease.
ECT is one of most effective treatments for depression, with the implementation of safer equipment and advancement of techniques such as modified ECT [153]. Mounting evidence from randomized controlled trial (RCT) and meta-analysis studies has shown that rTMS can treat depressive patients with safety [154]. Other promising treatments for depression have emerged, such as transcranial direct current stimulation (tDCS) [155], transcranial alternating current stimulation (tACS)[156], vagal nerve stimulation [157], deep brain stimulation [158] , and light therapy [159], but some of them are still experimental to some extent and have not been widely used. For example, compared to tDCS, tACS displays less sensory experience and adverse reactions with weak electrical current in a sine-wave pattern, but the evidence for the efficacy of tACS in the treatment of depression is still limited [160]. Alexander et al. recently demonstrated that there was no difference in efficacy among different treatments (sham, 10-Hz and 40-Hz tACS). However, only the 10-Hz tACS group had more responders than the sham and 40-Hz tACS groups at week 2 [156]. Further RCT studies are needed to verify the efficacy of tACS. In addition, the mechanism of the effect of neuromodulation therapy on depression needs to be further investigated.
Precision Medicine for Depression
Optimizing the treatment strategy is an effective way to improve the therapeutic effect on depression. However, each individual with depression may react very differently to different treatments. Therefore, this raises the question of personalized treatment, that is, which patients are suitable for which treatment. Over the past decade, psychiatrists and psychologists have focused on individual biomarkers and clinical characteristics to predict the efficiency of antidepressants and psychotherapies, including genetics, peripheral protein expression, electrophysiology, neuroimaging, neurocognitive performance, developmental trauma, and personality [161]. For example, Bradley et al. recently conducted a 12-week RCT, which demonstrated that the response rate and remission rates of the pharmacogenetic guidance group were significantly higher than those of the non-pharmacogenetic guidance group [162].
Subsequently, Greden et al. conducted an 8-week RCT of Genomics Used to Improve Depression Decisions (GUIDED) on 1,167 MDD patients and demonstrated that although there was no difference in symptom improvement between the pharmacogenomics-guided and non- pharmacogenomics-guided groups, the response rate and remission rate of the pharmacogenomics-guided group increased significantly [163].
A recent meta-analysis has shown that the baseline default mode network connectivity in patients with depression can predict the clinical responses to treatments including cognitive behavioral therapy, pharmacotherapy, ECT, rTMS, and transcutaneous vagus nerve stimulation [164]. However, so far, the biomarkers that predict treatment response at the individual level have not been well applied in the clinic, and there is still a lot of work to be conducted in the future.
Future Perspectives
Although considerable progress has been made in the study of depression during a past decade, the heterogeneity of the disease, the effectiveness of treatment, and the gap in translational medicine are critical challenges. The main dilemma is that our understanding of the etiology and pathophysiology of depression is inadequate, so our understanding of depression is not deep enough to develop more effective treatment. Animal models still cannot fully simulate this heterogeneous and complex mental disorder. Therefore, how to effectively match the indicators measured in animals with those measured in genetic research or the development of new antidepressants is another important challenge.
Change history
17 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12264-021-00694-9
References
Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health 2020, 20: 173
Zhu S, Zhao L, Fan Y, Lv Q, Wu K, Lang X. Interaction between TNF-alpha and oxidative stress status in first-episode drug-naive schizophrenia. Psychoneuroendocrinology 2020, 114: 104595
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390: 1211–1259
Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W,et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res 2003, 12: 3–21
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013, 34: 119–138
Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018, 8: 2861
Liu J, Yan F, Ma X, Guo HL, Tang YL, Rakofsky JJ, et al. Prevalence of major depressive disorder and socio-demographic correlates: Results of a representative household epidemiological survey in Beijing, China. J Affect Disord 2015, 179: 74–81
Zhang YS, Rao WW, Cui LJ, Li JF, Li L, Ng CH, et al. Prevalence of major depressive disorder and its socio-demographic correlates in the general adult population in Hebei province, China. J Affect Disord 2019, 252: 92–98
Ma X, Xiang YT, Cai ZJ, Li SR, Xiang YQ, Guo HL, et al. Prevalence and socio-demographic correlates of major depressive episode in rural and urban areas of Beijing, China. J Affect Disord 2009, 115: 323–330
Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 2019, 6: 211–224
Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 2015, 54(37–44): e32
Wang F, Zhang QE, Zhang L, Ng CH, Ungvari GS, Yuan Z, et al. Prevalence of major depressive disorder in older adults in China: A systematic review and meta-analysis. J Affect Disord 2018, 241: 297–304
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature 2008, 455: 894–902
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006, 163: 1905–1917
Li Z, Zhang Y, Wang Z, Chen J, Fan J, Guan Y, et al. The role of BDNF, NTRK2 gene and their interaction in development of treatment-resistant depression: data from multicenter, prospective, longitudinal clinic practice. J Psychiatr Res 2013, 47: 8–14
Murphy BE. Steroids and depression. J Steroid Biochem Mol Biol 1991, 38: 537–559
Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008, 31: 464–468
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 2017, 22: 527–536
Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, et al. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry 2006, 60: 472–478
Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013, 18: 692–699
Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front Psychiatry 2019, 10: 974
Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijima K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology 2008, 33: 152–161
Mickey BJ, Ginsburg Y, Sitzmann AF, Grayhack C, Sen S, Kirschbaum C, et al. Cortisol trajectory, melancholia, and response to electroconvulsive therapy. J Psychiatr Res 2018, 103: 46–53
Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011, 73: 114–126
Aubry JM. CRF system and mood disorders. J Chem Neuroanat 2013, 54: 20–24
Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J Affect Disord 2020, 264: 527–534
Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 2012, 35: 47–56
Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 2008, 7: 426–437
Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2011, 35: 1558–1568
Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 2015, 20: 1057–1068
Chandley MJ, Szebeni A, Szebeni K, Crawford JD, Stockmeier CA, Turecki G, et al. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int J Neuropsychopharmacol 2014, 17: 1569–1578
Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 2013, 73: 1180–1188
Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments. Int J Neuropsychopharmacol 2019, 22: 119–135
Beurel E, Grieco SF, Amadei C, Downey K, Jope RS. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord 2016, 18: 473–480
Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 2008, 54: 577–587
Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102: 75–90
Ghosal S, Hare B, Duman RS. Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Curr Opin Behav Sci 2017, 14: 1–8
Fee C, Banasr M, Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol Psychiatry 2017, 82: 549–559
Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 2016, 37: 3337–3352
Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012, 17: 1130–1142
Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 2010, 13: 411–420
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 2017, 81: 886–897
Chen X, van Gerven J, Cohen A, Jacobs G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacol Sin 2019, 40: 571–582
Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 2016, 80: 457–468
Kolata SM, Nakao K, Jeevakumar V, Farmer-Alroth EL, Fujita Y, Bartley AF, et al. Neuropsychiatric phenotypes produced by GABA reduction in mouse cortex and hippocampus. Neuropsychopharmacology 2018, 43: 1445–1456
Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2475–2484
Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci 2012, 367: 2378–2381
Li K, Shen S, Ji YT, Li XY, Zhang LS, Wang XD. Melatonin augments the effects of fluoxetine on depression-like behavior and hippocampal BDNF-TrkB signaling. Neurosci Bull 2018, 34: 303–311
Zhang JJ, Gao TT, Wang Y, Wang JL, Guan W, Wang YJ, et al. Andrographolide exerts significant antidepressant-like effects involving the hippocampal BDNF system in mice. Int J Neuropsychopharmacol 2019, 22: 585–600
Wang JQ, Mao L. The ERK pathway: Molecular mechanisms and treatment of depression. Mol Neurobiol 2019, 56: 6197–6205
Chiou YJ, Huang TL. Serum brain-derived neurotrophic factors in taiwanese patients with drug-naive first-episode major depressive disorder: Effects of antidepressants. Int J Neuropsychopharmacol 2017, 20: 213–218
Youssef MM, Underwood MD, Huang YY, Hsiung SC, Liu Y, Simpson NR, et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol 2018, 21: 528–538
Kojima M, Matsui K, Mizui T. BDNF pro-peptide: physiological mechanisms and implications for depression. Cell Tissue Res 2019, 377: 73–79
Jeon SW, Kim YK. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J Neuroimmunol 2017, 313: 92–98
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2016, 16: 22–34
Zhao G, Liu X. Neuroimmune advance in depressive disorder. Adv Exp Med Biol 2019, 1180: 85–98
Li Z, Wang Z, Zhang C, Chen J, Su Y, Huang J, et al. Reduced ENA78 levels as novel biomarker for major depressive disorder and venlafaxine efficiency: Result from a prospective longitudinal study. Psychoneuroendocrinology 2017, 81: 113–121
Ambrosio G, Kaufmann FN, Manosso L, Platt N, Ghisleni G, Rodrigues ALS, et al. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 2018, 91: 132–141
Mao R, Zhang C, Chen J, Zhao G, Zhou R, Wang F, et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J Affect Disord 2018, 237: 65–72
Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019, 81: 24–40
Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 2018, 95: 43–49
Syed SA, Beurel E, Loewenstein DA, Lowell JA, Craighead WE, Dunlop BW, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron 2018, 99(914–924): e913
Li Z, Qi D, Chen J, Zhang C, Yi Z, Yuan C, et al. Venlafaxine inhibits the upregulation of plasma tumor necrosis factor-alpha (TNF-alpha) in the Chinese patients with major depressive disorder: a prospective longitudinal study. Psychoneuroendocrinology 2013, 38: 107–114
Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 2018, 55: 4195–4206
Innes S, Pariante CM, Borsini A. Microglial-driven changes in synaptic plasticity: A possible role in major depressive disorder. Psychoneuroendocrinology 2019, 102: 236–247
Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: Friend or foe? Mol Neurobiol 2017, 54: 8071–8089
Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci 2018, 12: 306
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017, 179: 223–244
Gonzalez-Arancibia C, Urrutia-Pinones J, Illanes-Gonzalez J, Martinez-Pinto J, Sotomayor-Zarate R, Julio-Pieper M, et al. Do your gut microbes affect your brain dopamine? Psychopharmacology (Berl) 2019, 236: 1611–1622
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol 2014, 20: 14105–14125
Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002, 122: 1140–1156
Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011: CD003460.
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 2017, 7: 43859
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016, 21: 786–796
Curtis K, Stewart CJ, Robinson M, Molfese DL, Gosnell SN, Kosten TR, et al. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur J Neurosci 2019, 50: 2446–2452
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 2009, 65: 263–267
Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr 2006, 43: 16–24
Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104: 132–142
Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2: 256–261
Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100: 213–222
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 2013, 36: 305–312
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol 2017, 232: 2359–2372
Diviccaro S, Giatti S, Borgo F, Barcella M, Borghi E, Trejo JL, et al. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology 2019, 99: 206–215
Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, et al. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98: 52–60
Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76: 197–205
Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 80: 309–321
Xie X, Yu C, Zhou J, Xiao Q, Shen Q, Xiong Z, et al. Nicotinamide mononucleotide ameliorates the depression-like behaviors and is associated with attenuating the disruption of mitochondrial bioenergetics in depressed mice. J Affect Disord 2020, 263: 166–174
Wang XL, Yuan K, Zhang W, Li SX, Gao GF, Lu L. Regulation of circadian genes by the MAPK pathway: Implications for rapid antidepressant action. Neurosci Bull 2020, 36: 66–76
Xie X, Shen Q, Yu C, Xiao Q, Zhou J, Xiong Z, et al. Depression-like behaviors are accompanied by disrupted mitochondrial energy metabolism in chronic corticosterone-induced mice. J Steroid Biochem Mol Biol 2020, 200: 105607
Zhang LF, Shi L, Liu H, Meng FT, Liu YJ, Wu HM, et al. Increased hippocampal tau phosphorylation and axonal mitochondrial transport in a mouse model of chronic stress. Int J Neuropsychopharmacol 2012, 15: 337–348
Flint J, Kendler KS. The genetics of major depression. Neuron 2014, 81: 1214
Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2009, 14: 359–375
Dunn EC, Brown RC, Dai Y, Rosand J, Nugent NR, Amstadter AB, et al. Genetic determinants of depression: recent findings and future directions. Harv Rev Psychiatry 2015, 23: 1–18
Major Depressive Disorder Working Group of the Psychiatric GC, Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013, 18: 497–511.
Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, et al. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry 2014, 76: 510–512
Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, et al. Psychiatric genomics: An update and an agenda. Am J Psychiatry 2018, 175: 15–27
Schwabe I, Milaneschi Y, Gerring Z, Sullivan PF, Schulte E, Suppli NP, et al. Unraveling the genetic architecture of major depressive disorder: merits and pitfalls of the approaches used in genome-wide association studies. Psychol Med 2019, 49: 2646–2656
Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 2016, 48: 624–633
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018, 50: 668–681
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019, 22: 343–352
Ormel J, Hartman CA, Snieder H. The genetics of depression: successful genome-wide association studies introduce new challenges. Transl Psychiatry 2019, 9: 114
Uchida S, Yamagata H, Seki T, Watanabe Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin Neurosci 2018, 72: 212–227
Yeshurun S, Hannan AJ. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol Psychiatry 2019, 24: 536–548
van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, et al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry 2013, 170: 1477–1486
Peng W, Chen Z, Yin L, Jia Z, Gong Q. Essential brain structural alterations in major depressive disorder: A voxel-wise meta-analysis on first episode, medication-naive patients. J Affect Disord 2016, 199: 114–123
Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: A systematic review and meta-analysis. Biol Psychiatry 2017, 82: 339–350
Maller JJ, Broadhouse K, Rush AJ, Gordon E, Koslow S, Grieve SM. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Mol Psychiatry 2018, 23: 1737–1744
Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 2016, 79: 282–292
Gryglewski G, Baldinger-Melich P, Seiger R, Godbersen GM, Michenthaler P, Klobl M, et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br J Psychiatry 2019, 214: 159–167
Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 2013, 38: 1068–1077
Liang S, Wang Q, Kong X, Deng W, Yang X, Li X, et al. White matter abnormalities in major depression biotypes identified by diffusion tensor imaging. Neurosci Bull 2019, 35: 867–876
Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry 2017, 76: 179–187
Brakowski J, Spinelli S, Dorig N, Bosch OG, Manoliu A, Holtforth MG, et al. Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res 2017, 92: 147–159
Dutta A, McKie S, Downey D, Thomas E, Juhasz G, Arnone D, et al. Regional default mode network connectivity in major depressive disorder: modulation by acute intravenous citalopram. Transl Psychiatry 2019, 9: 116
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry 2019, 9: 64
Muller VI, Cieslik EC, Serbanescu I, Laird AR, Fox PT, Eickhoff SB. Altered brain activity in unipolar depression revisited: Meta-analyses of neuroimaging studies. JAMA Psychiatry 2017, 74: 47–55
Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, et al. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord 2017, 207: 86–94
Godlewska BR, Browning M, Norbury R, Igoumenou A, Cowen PJ, Harmer CJ. Predicting treatment response in depression: The role of anterior cingulate cortex. Int J Neuropsychopharmacol 2018, 21: 988–996
Goldstein-Piekarski AN, Staveland BR, Ball TM, Yesavage J, Korgaonkar MS, Williams LM. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl Psychiatry 2018, 8: 57
Leaver AM, Vasavada M, Joshi SH, Wade B, Woods RP, Espinoza R, et al. Mechanisms of antidepressant response to electroconvulsive therapy studied with perfusion magnetic resonance imaging. Biol Psychiatry 2019, 85: 466–476
Miskowiak KW, Macoveanu J, Jorgensen MB, Stottrup MM, Ott CV, Jensen HM, et al. Neural response after a single ECT session during retrieval of emotional self-referent words in depression: A randomized, sham-controlled fMRI study. Int J Neuropsychopharmacol 2018, 21: 226–235
Du L, Liu H, Du W, Chao F, Zhang L, Wang K, et al. Stimulated left DLPFC-nucleus accumbens functional connectivity predicts the anti-depression and anti-anxiety effects of rTMS for depression. Transl Psychiatry 2018, 7: 3
Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety 2017, 34: 9–24
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016, 3: 472–480
Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 2015, 40: 2398–2408
Hou Z, Gong L, Zhi M, Yin Y, Zhang Y, Xie C, et al. Distinctive pretreatment features of bilateral nucleus accumbens networks predict early response to antidepressants in major depressive disorder. Brain Imaging Behav 2018, 12: 1042–1052
De Crescenzo F, Ciliberto M, Menghini D, Treglia G, Ebmeier KP, Janiri L. Is (18)F-FDG-PET suitable to predict clinical response to the treatment of geriatric depression? A systematic review of PET studies. Aging Ment Health 2017, 21: 889–894
Schmaal L, Pozzi E, T CH, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl Psychiatry 2020, 10: 172.
Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016, 21: 806–812
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017, 22: 900–909
Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res 2017, 86: 58–65
Renteria ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 2017, 7: e1116
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med 2020, 50: 1020–1031
de Kovel CGF, Aftanas L, Aleman A, Alexander-Bloch AF, Baune BT, Brack I, et al. No alterations of brain structural asymmetry in major depressive disorder: An ENIGMA consortium analysis. Am J Psychiatry 2019, 176: 1039–1049
Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp 2020.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry 2020.
van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry 2020, 25: 1511–1525
Lv X, Si T, Wang G, Wang H, Liu Q, Hu C, et al. The establishment of the objective diagnostic markers and personalized medical intervention in patients with major depressive disorder: rationale and protocol. BMC Psychiatry 2016, 16: 240
Fabbri C, Hosak L, Mossner R, Giegling I, Mandelli L, Bellivier F, et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J Biol Psychiatry 2017, 18: 5–28
Li Z, Zhang C, Fan J, Yuan C, Huang J, Chen J, et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study. Br J Psychiatry 2014, 205: 29–35
Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry 2015, 77: 223–235
Papakostas GI, Shelton RC, Kinrys G, Henry ME, Bakow BR, Lipkin SH, et al. Assessment of a multi-assay, serum-based biological diagnostic test for major depressive disorder: a pilot and replication study. Mol Psychiatry 2013, 18: 332–339
Kolovos S, Kleiboer A, Cuijpers P. Effect of psychotherapy for depression on quality of life: meta-analysis. Br J Psychiatry 2016, 209: 460–468
Park LT, Zarate CA Jr. Depression in the primary care setting. N Engl J Med 2019, 380: 559–568
Health N C C F M . Depression: The treatment and management of depression in adults (updated edition) 2010.
Qaseem A, Barry MJ, Kansagara D. Clinical Guidelines Committee of the American College of P. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the american college of physicians. Ann Intern Med 2016, 164: 350–359
Dodd S, Mitchell PB, Bauer M, Yatham L, Young AH, Kennedy SH, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: An international consensus statement. World J Biol Psychiatry 2018, 19: 330–348
Cristea IA, Naudet F. US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 2019, 6: 975–977
Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020, 265: 63–70
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry 2019, 176: 428–438
Turner EH. Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry 2019, 6: 977–979
Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 2017, 16: 472–486
Gill SP, Kellner CH. Clinical practice recommendations for continuation and maintenance electroconvulsive therapy for depression: Outcomes from a review of the evidence and a consensus workshop held in Australia in May 2017. J ECT 2019, 35: 14–20
McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry 2018, 79.
Chase HW, Boudewyn MA, Carter CS, Phillips ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry 2020, 25: 397–407
Alexander ML, Alagapan S, Lugo CE, Mellin JM, Lustenberger C, Rubinow DR, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry 2019, 9: 106
Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: Techniques, current modalities, and future challenges. Neurosci Bull 2016, 32: 115–126
Zhou C, Zhang H, Qin Y, Tian T, Xu B, Chen J, et al. A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 82: 224–232
Li X, Li X. The antidepressant effect of light therapy from retinal projections. Neurosci Bull 2018, 34: 359–368
Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: A systematic review. Ann Intern Med 2018, 168: 414–421
Kessler RC. The potential of predictive analytics to provide clinical decision support in depression treatment planning. Curr Opin Psychiatry 2018, 31: 32–39
Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demonstrating clinical utility. J Psychiatr Res 2018, 96: 100–107
Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res 2019, 111: 59–67
Long Z, Du L, Zhao J, Wu S, Zheng Q, Lei X. Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J Affect Disord 2020, 276: 62–68
Acknowledgments
This review was supported by the National Basic Research Development Program of China (2016YFC1307100), the National Natural Science Foundation of China (81930033 and 81771465; 81401127), Shanghai Key Project of Science & Technology (2018SHZDZX05), Shanghai Jiao Tong University Medical Engineering Foundation (YG2016MS48), Shanghai Jiao Tong University School of Medicine (19XJ11006), the Sanming Project of Medicine in Shenzhen Municipality (SZSM201612006), the National Key Technologies R&D Program of China (2012BAI01B04), and the Innovative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Ruan, M., Chen, J. et al. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 37, 863–880 (2021). https://doi.org/10.1007/s12264-021-00638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-021-00638-3