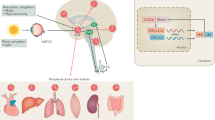
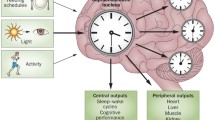
Abstract
Circadian rhythm is manifested by the behavioral and physiological changes from day to night, which is controlled by the pacemaker and its regulator. The former is located at the suprachiasmatic nuclei (SCN) in the anterior hypothalamus, while the latter is composed of clock genes present in all tissues. Circadian desynchronization influences normal patterns of day-night rhythms such as sleep and alertness cycles, rest and activity cycles. Parkinson’s disease (PD) exhibits diurnal fluctuations. Circadian dysfunction has been observed in PD patients and animal models, which may result in negative consequences to the homeostasis and even exacerbate the disease progression. Therefore, circadian therapies, including light stimulation, physical activity, dietary and social schedules, may be helpful for PD patients. However, the cellular and molecular mechanisms that underlie the circadian dysfunction in PD remain elusive. Further research on circadian patterns is needed. This article summarizes the existing research on the circadian rhythms in PD, focusing on the clinical symptom variations, molecular changes, as well as the available treatment options.

Similar content being viewed by others
References
Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 2014, 29: 1583–1590.
Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010, 1184: 15–54.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003, 24: 197–211.
McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A 2005, 102: 9377–9381.
Kawarai T, Kawakami H, Yamamura Y, Nakamura S. Structure and organization of the gene encoding human dopamine transporter. Gene 1997, 195: 11–18.
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry 2010, 68: 503–511.
Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience 2009, 158: 537–544.
Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Proc Natl Acad Sci U S A 2006, 103: 6386–6391.
Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci 2010, 30: 14046–14058.
Kovacikova Z, Sladek M, Bendova Z, Illnerova H, Sumova A. Expression of clock and clock-driven genes in the rat suprachiasmatic nucleus during late fetal and early postnatal development. J Biol Rhythms 2006, 21: 140–148.
Seron-Ferre M, Mendez N, Abarzua-Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, et al. Circadian rhythms in the fetus. Mol Cell Endocrinol 2012, 349: 68–75.
Torres-Farfan C, Rocco V, Monso C, Valenzuela FJ, Campino C, Germain A, et al. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 2006, 147: 4618–4626.
Gonzalez S, Moreno-Delgado D, Moreno E, Perez-Capote K, Franco R, Mallol J, et al. Circadian-related heteromerization of adrenergic and dopamine D(4) receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol 2012, 10: e1001347.
Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 2005, 49: 429–454.
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009, 24: 1641–1649.
Lee MA, Prentice WM, Hildreth AJ, Walker RW. Measuring symptom load in Idiopathic Parkinson’s disease. Parkinsonism Relat Disord 2007, 13: 284–289.
Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007, 22: 1623–1629.
van Hilten B, Hoff JI, Middelkoop HA, van der Velde EA, Kerkhof GA, Wauquier A, et al. Sleep disruption in Parkinson’s disease. Assessment by continuous activity monitoring. Arch Neurol 1994, 51: 922–928.
Kurtis MM, Rodriguez-Blazquez C, Martinez-Martin P, Group E. Relationship between sleep disorders and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2013, 19: 1152–1155.
Happe S, Schrodl B, Faltl M, Muller C, Auff E, Zeitlhofer J. Sleep disorders and depression in patients with Parkinson’s disease. Acta Neurol Scand 2001, 104: 275–280.
Chahine LM, Daley J, Horn S, Duda JE, Colcher A, Hurtig H, et al. Association between dopaminergic medications and nocturnal sleep in early-stage Parkinson’s disease. Parkinsonism Relat Disord 2013, 19: 859–863.
Suzuki K, Miyamoto M, Miyamoto T, Iwanami M, Hirata K. Sleep disturbances associated with Parkinson’s disease. Parkinsons Dis 2011, 2011: 219056.
Tan EK, Lum SY, Fook-Chong SM, Teoh ML, Yih Y, Tan L, et al. Evaluation of somnolence in Parkinson’s disease: comparison with age- and sex-matched controls. Neurology 2002, 58: 465–468.
Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology 2015, 85: 162–168.
Yi PL, Tsai CH, Lu MK, Liu HJ, Chen YC, Chang FC. Interleukin-1beta mediates sleep alteration in rats with rotenone-induced parkinsonism. Sleep 2007, 30: 413–425.
Lu CY, Yi PL, Tsai CH, Cheng CH, Chang HH, Hsiao YT, et al. TNF-NF-kappaB signaling mediates excessive somnolence in hemiparkinsonian rats. Behav Brain Res 2010, 208: 484–496.
Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson’s disease. Exp Neurol 2013, 243: 45–56.
Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2010, 75: 494–499.
Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009, 72: 1296–1300.
Sorensen GL, Mehlsen J, Jennum P. Reduced sympathetic activity in idiopathic rapid-eye-movement sleep behavior disorder and Parkinson’s disease. Auton Neurosci 2013, 179: 138–141.
Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 2007, 69: 1843–1849.
Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: a prospective study. Mov Disord 2012, 27: 720–726.
Luppi PH, Clement O, Valencia Garcia S, Brischoux F, Fort P. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med 2013, 14: 714–718.
Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, et al. Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol 2016, 15: 708–718.
Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, et al. Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol 2009, 219: 574–582.
Verhave PS, Jongsma MJ, Van den Berg RM, Vis JC, Vanwersch RA, Smit AB, et al. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. Sleep 2011, 34: 1119–1125.
van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1993, 56: 874–877.
van Hilten JJ, Middelkoop HA, Kerkhof GA, Roos RA. A new approach in the assessment of motor activity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1991, 54: 976–979.
Niwa F, Kuriyama N, Nakagawa M, Imanishi J. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton Neurosci 2011, 165: 195–200.
Pan W, Kwak S, Li F, Wu C, Chen Y, Yamamoto Y, et al. Actigraphy monitoring of symptoms in patients with Parkinson’s disease. Physiol Behav 2013, 119: 156–160.
Bonuccelli U, Del Dotto P, Lucetti C, Petrozzi L, Bernardini S, Gambaccini G, et al. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin Neuropharmacol 2000, 23: 28–33.
Nutt JG, Carter JH, Lea ES, Woodward WR. Motor fluctuations during continuous levodopa infusions in patients with Parkinson’s disease. Mov Disord 1997, 12: 285–292.
Piccini P, Del Dotto P, Pardini C, D’Antonio P, Rossi G, Bonuccelli U. Diurnal worsening in Parkinson patients treated with levodopa. Riv Neurol 1991, 61: 219–224.
Baier PC, Branisa P, Koch R, Schindehutte J, Paulus W, Trenkwalder C. Circadian distribution of motor-activity in unilaterally 6-hydroxy-dopamine lesioned rats. Exp Brain Res 2006, 169: 283–288.
Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol 2011, 232: 66–75.
Monville C, Torres EM, Pekarik V, Lane EL, Dunnett SB. Genetic, temporal and diurnal influences on L-dopa-induced dyskinesia in the 6-OHDA model. Brain Res Bull 2009, 78: 248–253.
Tong J, Qin LQ, Wang DJ. Mechanism of pineal and suprachiasmatic regulation on circadian rhythm of body temperature in rats. Space Med Med Eng (Beijing) 2000, 13: 101–103.
Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS One 2013, 8: e72661.
Cagnacci A, Bonuccelli U, Melis GB, Soldani R, Piccini P, Napolitano A, et al. Effect of naloxone on body temperature in postmenopausal women with Parkinson’s disease. Life Sci 1990, 46: 1241–1247.
Suzuki K, Miyamoto T, Miyamoto M, Kaji Y, Takekawa H, Hirata K. Circadian variation of core body temperature in Parkinson disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology 2007, 56: 172–179.
Lax P, Esquiva G, Esteve-Rudd J, Otalora BB, Madrid JA, Cuenca N. Circadian dysfunction in a rotenone-induced parkinsonian rodent model. Chronobiol Int 2012, 29: 147–156.
Rango M, Arighi A, Bonifati C, Bresolin N. Increased brain temperature in Parkinson’s disease. Neuroreport 2012, 23: 129–133.
Sumida K, Sato N, Ota M, Sakai K, Nippashi Y, Sone D, et al. Intraventricular cerebrospinal fluid temperature analysis using MR diffusion-weighted imaging thermometry in Parkinson’s disease patients, multiple system atrophy patients, and healthy subjects. Brain Behav 2015, 5: e00340.
Ejaz AA, Sekhon IS, Munjal S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson’s disease. Eur J Intern Med 2006, 17: 417–420.
Schmidt C, Berg D, Herting, Prieur S, Junghanns S, Schweitzer K, et al. Loss of nocturnal blood pressure fall in various extrapyramidal syndromes. Mov Disord 2009, 24: 2136–2142.
Berganzo K, Diez-Arrola B, Tijero B, Somme J, Lezcano E, Llorens V, et al. Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: are they related? J Neurol 2013, 260: 1752–1756.
Kallio M, Haapaniemi T, Turkka J, Suominen K, Tolonen U, Sotaniemi K, et al. Heart rate variability in patients with untreated Parkinson’s disease. Eur J Neurol 2000, 7: 667–672.
Devos D, Kroumova M, Bordet R, Vodougnon H, Guieu JD, Libersa C, et al. Heart rate variability and Parkinson’s disease severity. J Neural Transm (Vienna) 2003, 110: 997–1011.
Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J 2014, 55: 1297–1302.
Salsone M, Vescio B, Fratto A, Sturniolo M, Arabia G, Gambardella A, et al. Cardiac sympathetic index identifies patients with Parkinson’s disease and REM behavior disorder. Parkinsonism Relat Disord 2016, 26: 62–66.
Boulamery A, Simon N, Vidal J, Bruguerolle B. Effects of L-dopa on circadian rhythms of 6-OHDA striatal lesioned rats: a radiotelemetric study. Chronobiol Int 2010, 27: 251–264.
McDonald C, Newton JL, Burn DJ. Orthostatic hypotension and cognitive impairment in Parkinson’s disease: Causation or association? Mov Disord 2016, 31: 937–946.
McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res 2014, 39: 58–76.
Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 2004, 108: 17–40.
Struck LK, Rodnitzky RL, Dobson JK. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology 1990, 40: 467–470.
Van Hook MJ, Wong KY, Berson DM. Dopaminergic modulation of ganglion-cell photoreceptors in rat. Eur J Neurosci 2012, 35: 507–518.
Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol 2008, 6: e249.
Garfinkel D, Laudon M, Zisapel N. Improvement of sleep quality by controlled-release melatonin in benzodiazepine-treated elderly insomniacs. Arch Gerontol Geriatr 1997, 24: 223–231.
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in Parkinson’s disease. J Neural Transm Park Dis Dement Sect 1991, 3: 41–47.
Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of L-dopa. J Neural Transm Park Dis Dement Sect 1993, 5: 227–234.
Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol 2003, 26: 65–72.
Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med 2014, 15: 342–347.
Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 2014, 71: 463–469.
Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 2014, 71: 589–595.
Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, et al. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord 2016.
Bogaerts V, Theuns J, van Broeckhoven C. Genetic findings in Parkinson’s disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav 2008, 7: 129–151.
Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging 1997, 18: 285–289.
Tornhage CJ, Skogar O, Borg A, Larsson B, Robertsson L, Andersson L, et al. Short- and long-term effects of tactile massage on salivary cortisol concentrations in Parkinson’s disease: a randomised controlled pilot study. BMC Complement Altern Med 2013, 13: 357.
Mizobuchi M, Hineno T, Kakimoto Y, Hiratani K. Increase of plasma adrenocorticotrophin and cortisol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated dogs. Brain Res 1993, 612: 319–321.
Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J Neuroendocrinol 2011, 23: 519–524.
Langston JW, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol 1978, 3: 129–133.
Javoy-Agid F, Ruberg M, Pique L, Bertagna X, Taquet H, Studler JM, et al. Biochemistry of the hypothalamus in Parkinson’s disease. Neurology 1984, 34: 672–675.
Shannak K, Rajput A, Rozdilsky B, Kish S, Gilbert J, Hornykiewicz O. Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson’s disease and normal subjects. Brain Res 1994, 639: 33–41.
Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis 2008, 29: 381–390.
Politis M, Piccini P, Pavese N, Koh SB, Brooks DJ. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: an in vivo 11C-raclopride PET study. Exp Neurol 2008, 214: 112–116.
Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’–circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 2014, 10: 683–693.
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T. Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 2004, 5: 18.
Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A 2005, 102: 3111–3116.
Cai Y, Liu S, Sothern RB, Xu S, Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur J Neurol 2010, 17: 550–554.
Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci Lett 2011, 499: 186–188.
Gu Z, Wang B, Zhang YB, Ding H, Zhang Y, Yu J, et al. Association of ARNTL and PER1 genes with Parkinson’s disease: a case-control study of Han Chinese. Sci Rep 2015, 5: 15891.
Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology 2015, 16: 109–123.
Liu C, Chung M. Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders. Neurosci Bull 2015, 31: 141–159.
Liu HC, Hu CJ, Tang YC, Chang JG. A pilot study for circadian gene disturbance in dementia patients. Neurosci Lett 2008, 435: 229–233.
West RL, Lee JM, Maroun LE. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci 1995, 6: 141–146.
Lin Q, Ding H, Zheng Z, Gu Z, Ma J, Chen L, et al. Promoter methylation analysis of seven clock genes in Parkinson’s disease. Neurosci Lett 2012, 507: 147–150.
Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A 2015, 112: 7231–7236.
Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 2003, 301: 379–383.
Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O’Hara BF, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci U S A 2006, 103: 7118–7123.
Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103: 1009–1017.
Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005, 28: 395–409.
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 2001, 105: 683–694.
Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 2015, 125: 324–336.
Ait-Hmyed Hakkari O, Acar N, Savier E, Spinnhirny P, Bennis M, Felder-Schmittbuhl MP, et al. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB J 2016, 30: 3690-3701.
Kandalepas PC, Mitchell JW, Gillette MU. Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS One 2016, 11: e0157824.
Terman M. Evolving applications of light therapy. Sleep Med Rev 2007, 11: 497–507.
Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci 2004, 24: 4242–4249.
Paus S, Schmitz-Hubsch T, Wullner U, Vogel A, Klockgether T, Abele M. Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord 2007, 22: 1495–1498.
Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int 2007, 24: 521–537.
Willis GL, Moore C, Armstrong SM. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev Neurosci 2012, 23: 199–226.
Yamanaka Y, Hashimoto S, Masubuchi S, Natsubori A, Nishide SY, Honma S, et al. Differential regulation of circadian melatonin rhythm and sleep-wake cycle by bright lights and nonphotic time cues in humans. Am J Physiol Regul Integr Comp Physiol 2014, 307: R546–557.
Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, et al. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul Integr Comp Physiol 2015, 309: R1112–1121.
Yasumoto Y, Nakao R, Oishi K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS One 2015, 10: e0116476.
Klamroth S, Steib S, Devan S, Pfeifer K. Effects of Exercise Therapy on Postural Instability in Parkinson Disease: A Meta-analysis. J Neurol Phys Ther 2016, 40: 3–14.
Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med 2015, 23: 175–184.
Li F, Harmer P. Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with Parkinson disease, Oregon, 2008–2011. Prev Chronic Dis 2015, 12: E120.
Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 2015, 45: 171–179.
He Y, Cornelissen-Guillaume GG, He J, Kastin AJ, Harrison LM, Pan W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiol Int 2016, 33: 553–560.
Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S A 2016, 113: E1673–1682.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81471299), Jiangsu Provincial Special Program of Medical Science (BL2014042), Suzhou Clinical Key Disease Diagnosis and Treatment Technology Foundation (LCZX201304), the Plans for Graduate Research and Innovation in Colleges and Universities of Jiangsu Province, China (KYZZ15_0334) and Suzhou Medical Key Discipline Project, the Priority Academic Program Development of Jiangsu Higher Education Institutions, China (PAPD) and Suzhou Clinical Research Center of Neurological Disease (Szzx201503).
Author information
Authors and Affiliations
Corresponding author
Additional information
Siyue Li and Yali Wang contributed equally to this review.
Rights and permissions
About this article
Cite this article
Li, S., Wang, Y., Wang, F. et al. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull. 33, 62–72 (2017). https://doi.org/10.1007/s12264-016-0089-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-016-0089-7