Abstract
Adult-type hypolactasia (AtH or lactase non-persistence) is the physiological decline in lactase activity that manifests in majority of the world’s population after weaning. Recently, various single-nucleotide polymorphisms (SNPs) upstream of lactase gene (LCT) have been suggested to be associated with AtH or the lactase persistent trait in different human populations. C/T -13910 SNP was found be completely associated with AtH in Finnish population, and G/A -22018 SNP was found to be strongly, but not completely, associated with AtH. The aim of this study was to correlate G/A -22018 SNP with intestinal lactase activity in North Indian children. These children were also genotyped for C/T -13910 SNP. We also examined the differences in milk consumption and milk-related clinical symptoms in children with different genotypes of G/A -22018 and C/T -13910 SNPs. Intestinal biopsies were obtained from 231 children aged 2–16 years undergoing routine endoscopy for various abdominal complaints. The biopsies were assayed for lactase, sucrase, and maltase activities and genotyped for G/A -22018 and C/T -13910 SNPs using restriction fragment length polymorphism and DNA sequencing analysis. There was a significant correlation between lactase activity and different genotypes of G/A -22018 SNP. Children with G/G -22018 genotype had low lactase activity. With a reference value of <10 U/g protein (lactase activity) to be indicative of AtH, the sensitivity and specificity of genetic test based on G/A -22018 SNP was 94.4 and 94.1 %, respectively. Furthermore, the consumption of milk was lower in children with G/G -22018 genotype. Flatulence was the only symptom significantly more frequent among the children with G/G -22018 genotype compared to those with G/A and A/A -22018 genotypes. However, most of the children with G/G -22018 genotype seem to tolerate small amounts of milk without any significant difference in gastrointestinal symptoms from those with G/A and A/A -22018 genotypes.
Similar content being viewed by others
Introduction
Lactase or lactase-phlorizin hydrolase (LPH; EC 3.2.1.23.62) is an integral glycoprotein of the microvillus membrane of small intestinal epithelial cells (Mantei et al. 1988). Lactase is a bifunctional enzyme having lactase and glucosidase activities (Swallow 2003; Kaur et al. 2006a). Lactase activity is responsible for hydrolyzing the milk sugar lactose to glucose and galactose, and phlorizin hydrolase activity is responsible for hydrolyzing aryl and alkyl β-glycosides to phlorizin and β-glycosylceramides (Swallow 2003; Kaur et al. 2006a). Lactase is a critical enzyme for neonates that depend on their mother’s milk for nourishment. In the majority of human populations, lactase activity declines after weaning, a condition known as adult-type hypolactasia (AtH; or lactase non-persistence) (Wang et al. 1998). AtH, in many cases, leads to symptoms of lactose intolerance when a person consumes lactose containing food. The classic symptoms of lactose intolerance include abdominal bloating and pain, fullness, cramps, borborygmi, flatulence, loose stools, and diarrhea (Villako and Maaroos 1994; Gudmand-Hoyer 1994). AtH is controlled by an autosomal recessive single gene (Sahi et al. 1973). Lactase persistence (LP) is an autosomal dominant trait enabling the continued production of the enzyme lactase throughout adult life. In general, the frequency of LP is high in certain European populations, whereas it is low in the native populations of Australia, America, Africa, and Asia (Troelsen 2005).
Mechanisms proposed to explain the occurrence of AtH include decreased production of lactase (Lloyd et al. 1992), synthesis of an inactive high molecular weight lactase (Nsi-Emvo et al. 1987), defective post-translational modifications (Rings et al. 1992), and susceptibility of lactase to luminal proteases during weaning due to change in levels of sialyl and fucosyl transferases in the small intestine (Torres-Pinedo and Mahmood 1984; Mahmood and Torres-Pinedo 1985; Kaur et al. 2006b). A trimodal distribution in lactase activity, representing the homozygous recessive, heterozygous, and homozygous dominant subjects, has been reported in several studies (Ho et al. 1982; Flatz 1984; Harvey et al. 1995). The observation of the trimodal distribution of lactase activity implied that the AtH/LP trait most likely was due to cis-acting differences (SNPs) within or near the lactase gene (Wang et al. 1995). Initial confirmatory evidence for the cis-acting nature was obtained from mRNA expression studies (Wang et al. 1995). Independent evidence that onset of AtH is controlled by a cis-acting regulatory variant upstream of lactase gene (LCT) came from linkage studies in the Finnish families (Enattah et al. 2002). Sequence analysis of the 47 kb region upstream of LCT resulted in the identification of a total of 52 non-coding variants. Two of the single-nucleotide polymorphisms (SNPs), C/T -13910 (rs4988235) and G/A -22018 (rs182549), showed co-segregation with AtH (Enattah et al. 2002). Since the identification of the C/T -13910 and G/A -22018 SNPs associated with AtH, several studies have explored their functional significance (Olds and Sibley 2003; Troelsen et al. 2003). As a cis-acting element, -13910*T allele stimulates the activity of the lactase promoter in vitro by binding to Oct-1 transcription factor (Lewinsky et al. 2005). Therefore, a genetic test based on C/T -13910 variant as a means of identifying children genetically inclined toward developing AtH has been proposed (Rasinpera et al. 2004). Genetic testing may also complement hydrogen breath test (HBT) in several aspects, improving the diagnosis of adult-type hypolactasia (Schirru et al. 2007). The G/A-22018 polymorphism has been demonstrated to have little functional significance in the control of lactase gene expression (Olds and Sibley 2003; Troelsen et al. 2003). However, in Northern Chinese population, -22018*A allele may be associated with LP phenotype (Xu et al. 2010).
As genetic testing has gained attention in the field of lactose malabsorption, our aim was to study the usefulness of G/A -22018 SNP in improving the diagnosis of AtH among North Indian children. We investigated the disaccharidase activities in different age groups of children and correlated it with G/A -22018 SNP. We also examined the differences in milk consumption and milk-related clinical symptoms in children with different genotypes of the G/A -22018 SNP.
Subjects and methods
Patients
Patients were selected for inclusion in the study from those who presented for routine endoscopy at Department of Gastroenterology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh and were being investigated for unexplained diarrhea and abdominal pain. Intestinal biopsy specimens of more than 300 children (2–16 years) undergoing upper gastrointestinal endoscopy between March 2008 and March 2011 were analyzed for this study. Out of these samples, only 231 were included in this study. Samples showing sucrase activity <40 units per gram (U/g) protein and maltase activity <150 U/g protein, when lactase activity was <20 U/g protein, suggesting secondary causes of hypolactasia were excluded from the study. At the time of endoscopy, the families were asked to complete a questionnaire concerned with milk consumption and possible milk-related symptoms. Written informed consent to participate in the study was obtained from parents of the children. Most of the subjects were from Punjab, Haryana, Rajasthan, and adjoining regions of Northern India, which represented various ethnic groups residing in the region. The study was approved by Institute Ethics Committee PGIMER, Chandigarh.
Genotyping C/T -13910 and G/A -22018 SNPs (Restriction fragment length polymorphism; RFLP)
DNA was isolated from intestinal biopsy specimens using REDExtract-N-Amp Tissue PCR Kit (Sigma, Saint Louis, USA). C/T -13910 and G/A -22018 SNPs were analyzed through polymerase chain reaction (PCR) amplification followed by restriction enzyme digestion assay. PCR was carried out using REDExtract-N-Amp Tissue PCR Kit (Sigma, Saint Louis, USA). Primers used to amplify the fragment spanning C/T -13910 variant were: sense 5′-gga tgc act gct gtg atg ag-3′ and antisense 5′-ccc act gac cta tcc tcg tg-3′ (Buning et al. 2003). Each reaction was denatured at 95 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. Primers used to amplify the fragment spanning G/A -22018 variant were: sense 5′-aac agg cac gtg gag gag tt-3′ and antisense 5′-ccc acc tca gcc tct tga gt-3′ (Buning et al. 2003). Each reaction was denatured at 95 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 61 °C for 30 s, and 72 °C for 30 s. The reactions were given a final 10 min extension at 72 °C.
PCR products were quantitated and 500 ng of DNA was digested with 1 unit of BsmFΙ restriction enzyme (New England Biolabs, Foster City, CA) for C/T -13910 SNP and 2 units HhaΙ (New England Biolabs, Foster City, CA) for G/A -22018 SNP in a 15 μL reaction containing 1 × reaction buffer and BSA. The reactions were incubated for 4–5 h at 65 °C. A total of 7 μL of each reaction mixtures was electrophoresed through 2 % agarose gel and stained with ethidium bromide.
DNA sequencing
To confirm the PCR–RFLP results and discover other possible SNPs, PCR products were sequenced by using an automated DNA Sequencer (ABI 3730XL Genetic analyzer; Xcelris Genomics) with forward primer to read ≈400 base pairs in one direction. When necessary, the results were reconfirmed by sequencing the other strand with the reverse primer. Total of 140 PCR products were sequenced (70 each for C/T -13910 and G/A -22018 SNPs).
Assay of disaccharidase activities
Activities of intestinal disaccharidases (lactase, sucrase, and maltase) were reported in units where one unit is equal to the hydrolysis of 1 μmol of disaccharide in 1 min at 37 °C. The biopsy was weighed and homogenized on ice in 0.9 % NaCl. Homogenates were diluted (for biopsy of <4 mg weight, dilutions of 1:2, 1:5, and 1:20 were used for lactase, sucrase, and maltase, respectively), pipetted into test tube, mixed by vortexing, and incubated at 37 °C for 60 min. Thereafter, 300 μL of ice-cold glucose oxidase reagent was added to each test tube and incubated again at 37 °C for 60 min. Absorbance was measured at wavelength of 450 nm (Dahlqvist 1984). Specific activity was reported as units per gram (U/g) protein. The lactase/sucrase (L/S) ratio was also calculated for each sample. A cut off point of 10 U/g protein for lactase activity and 0.2 for L/S ratio was used for diagnosis of adult-type hypolactasia (Rasinpera et al. 2004).
Statistical analysis
All statistical analyses were performed using SPSS (version 15.0; The Predictive Analytics Company, SPSS Inc, Chicago, IL). Differences between groups were assessed by Mann–Whitney test, t test, and χ 2 test. The differences in lactase activity and L/S ratio between various genotypes of G/A -22018 SNP were tested with non-parametric Mann–Whitney test. Student’s t test was used to analyze differences in sucrase and maltase activities between various genotypes of G/A -22018 SNP. The differences in milk consumption and milk-related clinical symptoms between various genotypes of G/A -22018 SNP were tested with χ 2 test. P values < 0.05 were considered statistically significant. All variables were expressed as mean ± standard deviation (SD).
Results
Genotyping results (RFLP and DNA sequencing)
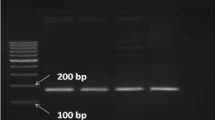
Amplification of the fragment spanning C/T -13910 SNP resulted in a 448 bp PCR product. For C/T -13910 SNP, digestion of the PCR product with restriction enzyme BsmFΙ revealed two fragments of ≈350 and ≈100 bp in case of the C/C -13910 genotype, and of ≈250 bp and ≈100/98 bp in case of T/T -13910 genotype (Fig. 1a). For C/T -13910 genotype, restriction digestion produced fragments of ≈350 bp, ≈250 bp, and ≈100/98 bp (Fig. 1a). For G/A -22018 SNP, PCR amplification also resulted in a 448-bp PCR product. Digestion with HhaΙ revealed fragments of ≈260 bp and ≈190 bp in case of G/G -22018 genotype and an undigested 448 bp fragment in case of A/A -22018 genotype (Fig. 1b).
Genotyping of individuals for C/T -13910 and G/A -22018 single-nucleotide polymorphisms by restriction digestion with BsmF1 and HhaΙ. Lane M DNA molecular weight marker (100 bp, Banglore Genei, India). Lane U Undigested PCR product. C/C, C/T, and T/T represent individuals with respective genotypes at -13910 position. G/G, G/A, and A/A represent individuals with respective genotypes at -22018 position
DNA sequencing was used to verify the PCR–RFLP results in 140 children (70 each for C/T -13910 and G/A -22018 SNPs). Sequencing data of all 140 samples for both C/T -13910 and G/A -22018 SNPs was in agreement with PCR–RFLP genotyping results. Furthermore, no polymorphism was present at positions -13915, -14010 and -13907 as reported for some other populations (Imtiaz et al. 2007; Ingram et al. 2007). Thus, the sequencing data showed that only C/T -13910 and G/A -22018 SNPs upstream of LCT are relevant polymorphisms associated with AtH/LP in Indian population.
Among the 231 children investigated in our study, 56.7 % (131/231) carried the C/C -13910 genotype, which has been associated with AtH, while 41.5 % (96/231) carried the C/T -13910 genotype and 1.7 % (4/231) the T/T -13910 genotype. For G/A -22018 SNP, 55.8 % (129/231) children showed the G/G -22018 genotype associated with AtH, 39.3 % (91/231) had the G/A -22018 genotype, and 4.7 % (11/231) had A/A -22018 genotype.
Correlation between G/A -22018 SNP and lactase activity
We examined the correlation of G/A -22018 SNP with disaccharidase activities in 231 intestinal biopsy specimens. The mean lactase activity in children with G/G -22018 genotype (n = 129) was 14.5 U/g protein and in those with G/A and A/A -22018 genotypes (n = 102) was 29.1 U/g protein (P < 0.001, Table 1). The mean L/S ratio for children with G/G -22018 genotype was 0.15 and for those with G/A and A/A -22018 genotypes was 0.31 (P < 0.001, Table 1). No significant correlation between sucrase activity and various genotypes of G/A -22018 SNP was detected. In case of maltase activity also, the correlation with various genotypes of G/A -22018 SNP was not significant. The G/G -22018 genotype was associated with very low lactase activity (<10 U/g protein) and low L/S ratio (<0.2) in majority of the children tested after 5 years of age (70/92,) and all the children above 8 years of age (52/52). Considering the enzyme activity as standard and using lactase activity below 10 U/g protein as cut off point for diagnosing AtH, the sensitivity, specificity, positive predictive value, and negative predictive value of genetic test based on G/A -22018 SNP in children >8 years of age were 94.4, 94.1, 96.2, and 91.4 %, respectively.
Correlation of G/A -22018 SNP with self-reported milk consumption and milk-related clinical symptoms
We examined the correlation of G/A -22018 SNP with self-reported milk consumption and milk-related clinical symptoms in 90/231 children. All the children examined were >8 years of age. Milk consumption was analyzed by a questionnaire, specially developed to analyze milk consumption and abdominal complaints (Anthoni et al. 2007). The subjects with the G/G -22018 genotype of AtH drank less milk as compared to those with the G/A -22018 or A/A -22018 genotypes. However, difference in milk intake between two groups of genotypes was not statistically significant (P = 0.25, χ 2 test). Among the children G/G -22018 genotype of AtH, 63 % (34/54) consumed milk ≤250 ml per day (approximately one cup or less of milk per day) and only 37 % (20/54) consumed milk >250 ml per day. For children with G/A -22018 or A/A -22018 genotypes, 38.9 % consumed milk ≤250 ml per day and 61.1 % consumed milk >250 ml per day. Gastrointestinal symptoms associated with AtH on consumption of milk, namely flatulence, bloating diarrhea, borborygmi, and constipation were observed among all genotype groups (Table 2). For milk consumption of ≤250 ml per day, no significant correlation between various genotypes of SNPs and milk-related clinical symptoms was observed (P = 0.77, 0.47, 0.82, 0.92, and 0.23 for flatulence, bloating, diarrhea, borborygmi, and constipation, respectively). When milk consumption was >250 ml per day, flatulence was the only symptom significantly more frequent among the subjects with G/G -22018 genotype (79 %) compared to those with G/A -22018 or A/A -22018 genotypes (36 %, P < 0.001, Table 2).
Discussion
AtH is an autosomal recessive gastrointestinal condition that is the result of decline in lactase activity of the intestinal lumen after weaning. In this study, we examined the correlation between G/A -22018 SNP and lactase activity in the intestinal biopsies of children aged 2–16 years. This may be helpful in improving the diagnosis of AtH in North Indian children. We also genotyped these children for C/T -13910 SNP. The usefulness of C/T -13910 SNP in improving the diagnosis of AtH among Indian population has already been reported in two studies (Babu et al. 2010; Kuchay et al. 2011). A study on Finnish pediatric population reported 100 % specificity and 93 % sensitivity of the C/T -13910 genotypes in predicting AtH from intestinal biopsy samples in patients >12 years of age (Rasinpera et al. 2004). The prevalence of the G/G -22018 genotype of AtH among 231 children participating in this study was 55.8 % which is almost equal to that of 56.7 % as observed for C/C -13910 genotype. The mean lactase activity in children with G/G -22018 genotype was 14.5 U/g protein. Among the 231 children, 131 had C/C -13910 genotype for C/T -13910 SNP, all but two of these children were also G/G homozygous for the G/A -22018 SNP. The remaining two were heterozygous for the G/A -22018 SNP. One of the two children heterozygous for the G/A -22018 SNP had low lactase activity (8.7 U/g protein) at the age of 10 years, the other had high lactase activity (30.2 U/g protein) at the same age. This is in agreement with the results of Ridefelt and Hakansson (2005) where in a study population of 44 Swedes and 7 non-Swedish individuals, all 51 samples from the patients undergoing lactose tolerance test showed full agreement between the C/T -13910 and G/A 22018 SNPs, that is, all patients with C/C -13910 genotype also had G/G -22018 genotype. Furthermore, analyses of samples from anonymous blood donors showed full agreement in 91 out of 93 cases, and the remaining two samples showed a combination of CC/AG and CT/AA genotypes at respective SNPs (Ridefelt and Hakansson 2005). Although lactase can show a patchy distribution in the small bowel, measurement of disaccharidase activity in intestinal biopsy remains one of the better procedures to classify the individual as LP or having AtH if secondary lactase deficiencies are excluded (Maiuri et al. 1992; Swallow 2003). In our study, G/A -22018 correlated well with the intestinal lactase activity in children that apparently had no secondary lactase deficiencies based on their L/S ratio. With a reference value of <10 U/g protein to be indicative of AtH, the sensitivity, specificity, positive predictive value, and negative predictive value of genetic test based on G/A -22018 SNP in Indian children >8 years were high. The values for sensitivity and specificity were almost equal to that observed for C/T -13910 SNP in Indian children > 8 years of age (Kuchay et al. 2011). This suggests that both -13910*C and -22018*G alleles may be useful in improving the diagnosis of AtH in Indian children. However, the genetic test based on C/T -13910 SNP has higher sensitivity and specificity.
In the present study, we found that the consumption of milk was lower in children with G/G -22018 genotype than with G/A -22018 and A/A -22018 genotypes. However, the difference in milk consumption between children with G/G -22018 genotype and those with G/A -22018 or A/A -22018 genotypes was not statistically significant. All the children examined were >8 years of age. Our results concerning milk consumption in children having genotypes associated with AtH are consistent with previously reported results (Anthoni et al. 2007; Laaksonen et al. 2009). Gastrointestinal symptoms associated with lactose intolerance, namely flatulence, bloating, borborygmi, and diarrhea, were found among all genotype groups. For consumption of ≤250 ml/day milk (approximately one cup or less of milk per day), the frequency of gastrointestinal symptoms was not statistically significant for all genotype groups (Table 2). Most of the children with G/G -22018 genotype seem to tolerate small amounts of milk (≤250 ml/day milk) without any significant difference in gastrointestinal symptoms from those with G/A -22018 and A/A -22018 genotypes. This is in agreement with the results that lactose intake if limited to the equivalent of 240 ml of milk or less a day, symptoms are likely to be negligible and the use of lactose-digestive aids unnecessary (Suarez et al. 1995; Suarez et al. 1997). For consumption of >250 ml/day milk, increase in the frequency of gastrointestinal symptoms was more for individuals with G/G -22018 genotype; however, flatulence was the only symptom significantly more frequent among the subjects with G/G -22018 genotype compared to those with G/A -22018 and A/A -22018 genotypes.
In conclusion, these findings suggest that -22018*G allele may be useful in improving the diagnosis of AtH in North Indian children. As -22018*G allele is associated with low lactase activity and low L/S ratio in children >8 years of age, it may be used to predict AtH in North Indian population. However, the present scenario about genetic testing for AtH is confusing and many new variants associated with AtH/LP have been identified in various human populations. Recent studies have revealed several new sequence variants upstream of lactase promoter, which are associated with AtH/LP in different populations (Ingram et al. 2007; Tishkoff et al. 2007; Enattah et al. 2008). In East Africa, T/G -13915 and C/G -13907 variants are significantly associated with LP, and G/C -14010 variant has borderline association (Ingram et al. 2007; Tishkoff et al. 2007; Enattah et al. 2008). One of these, T/G -13915, was also shown to be associated with high lactase expression in Saudi Arabia (Imtiaz et al. 2007). In our study, no such polymorphisms were present at positions -13915, -14010, and -13907. Thus, in North India, only C/T -13910 and G/A -22018 SNPs may be associated with AtH/LP in majority of the population. Furthermore, to elevate the diagnostic potential of genetic testing, secondary lactase deficiencies and irritable bowel syndrome need to be excluded. Also, the onset of hypolactasia in different populations can vary from 2 years of age to 21 years and the age after which the genetic test should be applied may also vary accordingly (Wang et al. 1998; Rasinpera et al. 2004). We would suggest genetic testing in combination with non-invasive tests like HBT for the screening of AtH. In subjects with negative HBT, genetic test may provides an unambiguous result permitting the exclusion of false negative results and avoiding the execution of further tests (Schirru et al. 2007). In our study, the difference in consumption of milk between children with G/G -22018 genotype and those with G/A -22018 and A/A -22018 genotypes was not statistically significant. Also, there was no significant correlation between various genotypes of G/A -22018 SNP and milk-related gastrointestinal symptoms in children that consumed small amount of milk. Thus, if the consumption of milk by individuals with G/G -22018 genotype is restricted to one cup of milk per day, the milk-related gastrointestinal symptoms are likely to be negligible.
References
Anthoni SR, Rasinpera HA, Kotamies AJ et al (2007) Molecularly defined adult-type hypolactasia among working age people with reference to milk consumption and gastrointestinal symptoms. World J Gastroenterol 28:1230–1235
Babu J, Kumar S, Babu P, Prasad JH, Ghoshal UC (2010) Frequency of lactose malabsorption among healthy southern and northern Indian populations by genetic analysis and lactose hydrogen breath and tolerance tests. Am J Clin Nutr 91:140–146
Buning C, Ockenga J, Kruger S et al (2003) The C/C(-13910) and G/G(-22018) genotypes for adult-type hypolactasia are not associated with inflammatory bowel disease. Scand J Gastroenterol 38:538–542
Dahlqvist A (1984) Assay of intestinal disaccharidases. Scand J Clin Lab Invest 44:169–172
Enattah NS, Sahi T, Savilahti E et al (2002) Identification of a variant associated with adult-type-hypolactasia. Nat Genet 30:233–237
Enattah NS, Jensen TG, Nielsen M, Lewinski R, Kuokkanen M, Rasinpera H et al (2008) Independent introduction of two lactase-persistence alleles into human populations reflects different history of adaptation to milk culture. Am J Hum Genet 82:57–72
Flatz G (1984) Gene-dosage effect on intestinal lactase activity demonstrated in vivo. Am J Hum Genet 36:306–310
Gudmand-Hoyer E (1994) The clinical significance of disaccharide maldigestion. Am J Clin Nutr 59:735S–741S
Harvey CB, Wang Y, Hughes LA, Swallow DM, Thurrell WP, Sams VR et al (1995) Studies on the expression of intestinal lactase in different individuals. Gut 36:28–33
Ho MW, Povey S, Swallow D (1982) Lactase polymorphism in adult British natives: estimating allele frequencies by enzyme assays in autopsy samples. Am J Hum Genet 34:650–657
Imtiaz F, Savilahti E, Sarnesto A et al (2007) The T/G 13915 variant upstream of the lactase gene (LCT) is the founder allele of lactase persistence in an urban Saudi population. J Med Genet 44:e89
Ingram CJE, Elamin MF, Mulcare CA et al (2007) A novel polymorphism associated with lactose tolerance in Africa: multiple causes for lactase persistence? Hum Genet 120:779–788
Kaur K, Mahmood S, Mahmood A (2006a) Hypolactasia as a molecular basis of lactose intolerance. Indian J Biochem Biophys 43:267–274
Kaur K, Mahmood S, Mahmood A (2006b) Susceptibility of lactase to luminal proteases in developing rat intestine. Ind J Gastroenterol 25:179–181
Kuchay RA, Thapa BR, Mahmood A, Mahmood S (2011) Effect of C/T −13910 cis-acting regulatory variant on expression and activity of lactase in Indian children and its implication for early genetic screening of adult-type hypolactasia. Clin Chim Acta 412:1924–1930
Laaksonen MM, Mikkila V, Rasanen L et al (2009) Genetic lactase non-persistence, consumption of milk products and intakes of milk nutrients in Finns from childhood to young adulthood. Br J Nutr 102:8–17
Lewinsky RH, Jensen TG, Moller J, Stensballe A, Olsen J, Troelsen JT (2005) T-13910 DNA variant associated with lactase persistence interacts with Oct-1 and stimulates lactase promoter activity in vitro. Hum Mol Genet 14:3945–3953
Lloyd M, Mevissen G, Fischer M et al (1992) Regulation of intestinal lactase in adult hypolactasia. J Clin Invest 89:524–529
Mahmood A, Torres-Pinedo R (1985) Effect of hormone administration on the sialylation and fucosylation of intestinal microvillus membranes of suckling rats. Pediatr Res 19:899–902
Maiuri L, Rossi M, Raia V, D’Auria S, Swallow D, Quaroni A et al (1992) Patchy expression of lactase protein in adult rabbit and rat intestine. Gastroentology 103(6):1739–1746
Mantei N, Villa M, Enzler T et al (1988) Complete primary structure of human and rabbit lactase-phlorizin hydrolase: implications for biosynthesis, membrane anchoring and evolution of the enzyme. EMBO J 7:2705–2713
Nsi-Emvo E, Launay JF, Raul F (1987) Is adult type hypolactasia in intestine of mammals related to changes in the intracellular processing of lactase? Cell Mol Biol 33:335–344
Olds LC, Sibley E (2003) Lactase persistence DNA variant enhances lactase promoter activity in vitro: functional role as a cis regulatory element. Hum Mol Genet 12:2333–2340
Rasinpera H, Savilahti E, Enattah NS et al (2004) A genetic test which can be used to diagnose adult-type hypolactasia in children. Gut 53:1571–1576
Ridefelt P, Hakansson LD (2005) Lactose intolerance: lactose tolerance test versus genotyping. Scand J Gastroenterol 40:822–826
Rings EH, De Boer PA, Moorman AF et al (1992) Lactase gene expression during early development of rat small intestine. Gastroenterology 103:1154–1161
Sahi T, Isokoski M, Jussila J, Launiala K, Pyorala K (1973) Recessive inheritance of adult type lactose malabsorption. Lancet 2:823–826
Schirru E, Corona V, Usai Satta P, Scarpa M, Oppia F, Loriga F et al (2007) Genetic testing improves the diagnosis of adult type hypolactasia in the Mediterranean population of Sardinia. Eur J Clin Nutr 61:1220–1225
Suarez FL, Savaiano DA, Levitt MD (1995) A comparison of symptoms after the consumption of milk or lactose hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 333:1–4
Suarez FL, Savaiano D, Arbisi P, Levitt MD (1997) Tolerance to the daily ingestion of two cups of milk by individuals claiming lactose intolerance. Am J Clin Nutr 65:1502–1506
Swallow DM (2003) Genetics of lactase persistence and lactose intolerance. Annu Rev Genet 37:197–219
Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS et al (2007) Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 39:31–40
Torres-Pinedo R, Mahmood A (1984) Postnatal changes in biosynthesis of microvillar membrane glycans of rat small intestine I. Evidence of a developmental shift from terminal sialylation to fucosylation. Biochem Biophys Res Commun 125:546–555
Troelsen JT (2005) Adult-type hypolactasia and regulation of lactase expression. Biochim Biophys Acta 1723:19–32
Troelsen JT, Olsen J, Moller J, Sjostrom H (2003) An upstream polymorphism associated with lactase persistence has increased enhancer activity. Gastroenterology 125:1686–1694
Villako K, Maaroos H (1994) Clinical picture of hypolactasia and lactose intolerance. Scand J Gastroenterol Suppl 202:36–54
Wang Y, Harvey CB, Pratt WS, Sams VR, Sarner M, Rossi M et al (1995) The lactase persistence/non-persistence polymorphism is controlled by a cis-acting element. Hum Mol Genet 4:657–662
Wang Y, Harvey CB, Hollox EJ et al (1998) The genetically programmed down-regulation of lactase in children. Gastroenterology 114:1230–1236
Xu L, Sun H, Zhang X et al (2010) The -22018A allele matches the lactase persistence phenotype in northern Chinese populations. Scand J Gastroenterol 45:168–174
Acknowledgments
We thank the children and their parents for participating in this study. We acknowledge Dr. Sadhna Lal and the supporting staff of Gastroenterology Department for helping in the collection of samples. We are thankful to Dr. Rosa M Munoz Xicola, University of Illinois at Chicago, for helping with DNA sequence analysis. We are thankful to all those involved in statistical analysis. We are thankful to the reviewers for their valuable suggestions regarding this manuscript. Laboratory of Safrun Mahmood was supported by Department of Biotechnology (DBT), New Delhi, India. Raja Amir was supported by Junior Research Fellowship, University Grants Commission, New Delhi, India.
Conflict of interest
None of the authors reported any personal or financial conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuchay, R.A.H., Anwar, M., Thapa, B.R. et al. Correlation of G/A -22018 single-nucleotide polymorphism with lactase activity and its usefulness in improving the diagnosis of adult-type hypolactasia among North Indian children. Genes Nutr 8, 145–151 (2013). https://doi.org/10.1007/s12263-012-0305-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12263-012-0305-7