Abstract
The female pelvis has a complex anatomy, and benign adnexal diseases can present as malignant ovarian masses clinically and radiologically. Between 1 June 2011 and 28 September 2022, we included in this study all the patients who were diagnosed initially with suspicious ovarian masses in the Department of Surgical Oncology, Oncology Center, Mansoura University, Egypt, and after surgical exploration revealed pelvic inflammatory disease and we assessed their diagnostic, operative, and postoperative outcomes. In this case series we had 41 patients of a total of 803 cases with suspicious adnexal mass that revealed pelvic inflammatory disease after surgical exploration, abdominal pain was the common presentation in 53.7% of the cases, and low-grade fever was reported in seven cases. The serum cancer antigen 125 was elevated in 70.7% of the cases. Unilateral adnexal mass was found in 30 cases and 11 cases had bilateral adnexal masses. We followed up on the patients within 47 months (range 12–88 months); recurrent pelvic inflammatory disease has developed in one case after 62 months and ovarian cancer has developed in another case after 80 months. We concluded that benign adnexal masses such as tubo-ovarian abscess secondary to pelvic inflammatory disease should be considered a differential diagnosis in patients with radiological suspicious adnexal masses when the tumor markers such as cancer antigen 125 are normal or mildly elevated, especially in premenopausal women, and the clinical presentations are not specific to either benign or malignant adnexal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of adnexal masses in asymptomatic women is 0.17 to 5.9% and in symptomatic women is 7.1 to 12%; less than 25% of these adnexal masses are diagnosed as malignant masses [1]. Malignant and benign adnexal diseases have the same presentation, such as non-specific pelvic pain, pelviabdominal mass, and vaginal hemorrhage, and imaging plays an important role in the diagnosis of these lesions and assessing their origins [2, 3]. Pelvic inflammatory disease (PID) and chronic tubo-ovarian abscesses are often misdiagnosed as ovarian cancer because of their solid component and high vascularity. Many cases with presumed benign or malignant adnexal masses were found to be chronic tubo-ovarian abscesses [4, 5], even with the absence of the clinical presentations that cope with an inflammatory process.
Pelvic inflammatory disease includes inflammation of the endometrium, fallopian tube, and pelvic peritoneum and formation of tubo-ovarian abscess, which is caused by microorganisms arising from the lower genital tract [6]. A tubo-ovarian abscess is a common complication of pelvic inflammatory disease (PID) and occurs in 15% of the cases, and it rarely occurs in sexually inactive girls [7]. The female pelvis has a complex anatomy and benign adnexal masses mimic ovarian cancer on imaging, which accounts for 1.6% of all female cancers and is the leading cause of death from gynecologic cancers [8]. Ultrasound (US) is the primary radiologic modality used in the assessment of any pelvic mass, and magnetic resonance imaging (MRI) is the most accurate method for further characterization of pelvic masses with its superior spatial resolution and soft tissue contrast [9]. We are reporting patients presented with suspicious adnexal masses clinically and radiologically to a cancer center that revealed pelvic inflammatory disease after surgical intervention with analysis of its diagnostic criteria, and operative, and postoperative outcomes.
Patients and Methods
This case series was done between 1 June 2011 and 28 September 2022, in the Department of Surgical Oncology, Oncology Center, Mansoura University, Egypt. We collected and analyzed the patients’ data from our database registry system. We included all the patients who were diagnosed initially with ovarian cancer radiologically, and with elevated serum cancer antigen (CA) 125, and after surgical exploration revealed pelvic inflammatory disease (PID). We have excluded patients with suspicious adnexal masses and confirmed ovarian cancer pathologically. The clinical presentations of suspicious adnexal masses including abdominal pain, enlargement, and pelvic pain were reported and analyzed. The clinical manifestations relevant to pelvic inflammatory disease including fever, night sweats, vaginal discharge, and elevated leucocytic count were collected and analyzed after confirmation of its diagnosis postoperatively. A preoperative contrast-enhanced chest and abdominal computed tomography (CT) scan was done for disease diagnosis and staging if there is liver, lung metastasis, or ascites in cases suspected to be ovarian cancer. Pelvic magnetic resonance imaging (MRI) with contrast was used for adnexal mass characterization with its solid component and contrast enhancement, and tumor markers such as cancer antigen (CA) 125, carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), and alpha-fetoprotein were used to confirm the diagnosis of ovarian cancer and used for follow-up. The operative approaches and the type of operation were reported including the operative time and complications. Postoperative outcomes including oral intake, postoperative morbidities according to Clavien-Dindo (CD) classification, hospital stay, and postoperative pathological examination were reported. The patients were followed up clinically, by imaging and with tumor markers.
Statistical Analysis
Data were analyzed using Statistical Package for Scientific Studies (SPSS) v26.0 (IBM Corp., Chicago, IL, USA) on MacOS v11.9. Qualitative data were described using numbers and percentages. Quantitative data were described using medians for non-parametric data and means and Standard Deviation (SD) for parametric data, after testing normality using the Kolmogorov-Smirnov test.
Results
We had 41 patients with suspicious adnexal masses that revealed pelvic inflammatory disease (PID) after surgical exploration; our patients had a mean age of 46.32 years (Table 1). The body mass index (BMI) was 34.66 kg/m2, 23 cases were premenopausal, and 18 cases were postmenopausal. Twenty patients have used an intrauterine device (IUD) as a method of contraception, 15 patients have used oral contraception, one patient has used a subcutaneous capsule (Implanon), and four patients have used injectable contraception. Thirty patients had previous abdominal surgeries; 20 patients had a previous cesarean section, four patients with previous appendectomy, four patients with previous cholecystectomy, and two patients with previous paraumbilical hernioplasty. The diagnostic criteria are reported in Table 2; abdominal pain was the common presentation in 53.7% of the cases, low-grade fever was reported in seven cases, and one case was complaining of night sweats. Vaginal discharge was reported in eight cases, and the leucocytic count was elevated in five cases with a median count of 8.6 mg/dL. The serum cancer antigen (CA) 125 was elevated in 70.7% of the cases (14 postmenopausal cases had CA125 > 35 U/mL and six premenopausal cases had CA125 > 200 U/mL), carcinoembryonic antigen (CEA) was elevated in 7 cases, lactate dehydrogenase (LDH) was elevated in two cases, and alpha-fetoprotein was elevated in three cases. Ascites was reported in 17 cases (14 cases with mild ascites and three cases with moderate ascites). Unilateral adnexal mass was found in 30 cases and 11 cases had bilateral adnexal masses.
The laparoscopic assessment was done in 11 cases, 30 cases had laparotomy, and conversion from laparoscopy to laparotomy was done in four cases (Table 3). Intraoperative ascites was found in 12 cases and their cytological examination was reactive. The intraoperative frozen section was done in 20 cases; 19 cases had salpingo-oophorectomy (16 unilateral, and 3 bilateral), and one case had temporary ileostomy due to extensive adhesion causing intestinal obstruction; the frozen section in these cases revealed tubo-ovarian abscess or chronic suppuration. We had 15 cases in this study that underwent excision and deroofing of the adnexal masses as they had extensive adhesions, and pus was drained intraoperatively, and the postoperative pathology and culture were inflammatory with non-specific organism infection. Total abdominal hysterectomy, omentectomy, and salpingo-oophorectomy were performed in six postmenopausal cases without frozen section examination and the postoperative pathology revealed pelvic inflammatory disease. Appendectomy was done in a case with unilateral salpingo-oophorectomy as the appendix was adherent to the ipsilateral ovary.
The operative time had a mean duration of 122.8 min, and the intraoperative estimated blood loss was about 68.66 mL, and intraoperative blood transfusion was needed in two cases. There were extensive adhesions in 63.4% that led to intraoperative complications in four cases: two cases with intestinal tear and repair (one case needed temporary ileostomy after optimal debulking), one case with a ureteric injury that needed repair, and one case with cyst rupture and had peritoneal lavage. The patients started oral on the first postoperative day (POD 1) (range 0–8 days), one case had ICU admission, and the median duration of the hospital stay was 3 days (range 1–22 days). Broad-spectrum antibiotics were administered from the day of surgery and after confirmation of the diagnosis. A postoperative complication of Clavien-Dindo (CD) grade II was found in five cases (Table 4) in the form of wound infection that necessitated antibiotic therapy and three cases had Clavien-Dindo (CD) grade III complications (one case with a gluteal abscess that was drained and two cases with an intestinal leak that needed temporary fecal diversion). We followed up with the patients within 47 months (range 12–88 months); recurrent pelvic inflammatory disease developed in one case after 62 months and ovarian cancer developed in another case after 80 months.
Discussion
In this case series, we evaluated pelvic inflammatory disease (PID) cases that resembled malignant ovarian masses clinically, laboratory, and in the radiologic features. A tubo-ovarian abscess is a severe sequel of pelvic inflammatory disease (PID) after inadequate diagnosis and treatment, and it forms a chronic inflammatory mass that mimics a malignant ovarian mass [10]. Ovarian cancer is rare at a young age, especially under the age of 30, and risk increases with aging, and its peak incidence is between 50 and 70 years [11]. Tubo-ovarian abscess and PID are diseases of premenopausal women (30–50 years) and rarely occur in young or postmenopausal women. The mean age of our patients was 46.32 years and 56.1% of them were premenopausal. Pelvic inflammatory disease (PID) can develop rarely in virgins, and it was diagnosed in 22 virgin cases reported in 20 case reports [12]. We had only one virgin case in the current study; pelvic inflammatory disease is a disease of sexually active women and has many causes such as an infected partner, previous history of pelvic inflammatory disease, the type of contraceptive method, the use of the intrauterine device (IUD), and tubal ligation [13]. In virgin women, several studies suggested the source of this infection is from the lower genital tract (most accepted), urinary tract, gastrointestinal tract, or skin wound. So tubo-ovarian abscess and pelvic inflammatory disease (PID) could be a differential diagnosis in a virgin case with suspicious adnexal mass and elevated CA125.
Clinical presentations and laboratory investigations play an important role in the diagnosis of pelvic inflammatory disease (PID) and ovarian cancer; however, most adnexal tumors have non-specific gynecologic complaints that can mimic tubo-ovarian abscesses or other para-ovarian masses. Abdominal pain was the most common presentation in the current study in 53.7% of the cases that was non-specific to any gynecologic disease, while 19.5% of the cases had pelvic pain. Most studies have included symptoms that raised the likelihood of ovarian cancer such as abdominal distension and pelvic pain [14]. Some other documents also reported symptoms such as fatigue, nausea, back pain, and urinary manifestations as suspicious presentations of ovarian cancers [15]. Fever was reported in seven cases and was a low grade that did not indicate any inflammatory process and the leucocytic count was mildly elevated in five cases. These manifestations made us not suspect any inflammatory process with suspicious adnexal mass in imaging and elevated CA125. No cases in the current study have fulfilled the pelvic inflammatory disease (PID) diagnostic criteria in 2015 by the Centers for Disease Control and Prevention (CDC) [16]. Another study reported that 40% of pelvic inflammatory disease patients had normal temperature and leucocytic count [17].
The serum cancer antigen (CA) 125 was elevated in 70.7% of the cases in the current study with the highest level of 501 U/mL, and we had 14 postmenopausal cases and 17 premenopausal cases with elevated serum CA125, and high serum CA125 is known to be associated with ovarian epithelial malignant tumors [18]. However, serum CA125 is elevated sometimes physiologically and in other non-malignant conditions such as endometriosis and pelvic inflammatory disease (PID) as the irritation or inflammation of the serous lining of the pelvis or the abdominal cavity by these diseases is associated with an elevation in CA125 levels. About 5% of healthy women have CA125 above 35 U/mL, and only 0.1% of them have CA125 above 100 U/mL. The cutoff value for CA125 in postmenopausal women is 35 U/mL, while this cutoff can produce false positive results in premenopausal women as it is elevated in several benign conditions. Therefore, the American College of Obstetrics and Gynecology has suggested that any premenopausal women with pelvic masses and a CA125 level of ≥ 200 U/mL should visit an oncologist [19]. In the current study, 14 postmenopausal cases had CA125 > 35 U/mL and six premenopausal cases had CA125 > 200 U/mL
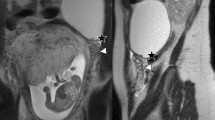
Contrast-enhanced pelvic magnetic resonance imaging (MRI) was performed for adnexal mass characterization, and any adnexal mass with thick ill-defined regular or irregular walls with contrast enhancement and a fluid content are typical findings of tubo-ovarian abscess [20]. However, pelvic inflammatory disease (PID) with tubo-ovarian abscess shows sometimes inflammatory process with infiltration, with ill-defined borders and invasion into the nearby structures, mimicking aggressive ovarian malignancy. Therefore, differentiation of tubo-ovarian abscess and ovarian cancer is challenging when clinical findings and leukocytosis are non-specific and associated with atypical MRI features. Fallopian tube dilatation (salpingitis or pyosalpinx) is commonly found in pelvic inflammatory disease cases, and not common in ovarian cancer [21]. So, any adnexal mass with complex criteria and dilated fallopian tube raises the diagnosis of pelvic inflammatory disease with tubo-ovarian abscess [10]. Another study reported that 82.4% of their patients with tubo-ovarian abscesses presented pseudo-solid-like signal intensity on T1- and T2-weighted images with high signal intensity on diffusion-weighted images (DWI) and low apparent diffusion coefficient (ADC) values, mimicking the solid components of ovarian tumors. Therefore, it is difficult to differentiate the solid parts of adnexal tumors from pus that has pseudo-solid-like signal intensity, with the non-contrast MRI and DWI [17].
The patients in the current study were admitted for surgical operation as suspicious adnexal masses; 11 cases had a laparoscopic assessment and 30 cases had laparotomy. These patients were found to have pelvic inflammatory disease either intraoperatively (by pus drainage from the adnexa or the frozen section) or postoperatively by the pathological examination. A study has reported five cases that were preoperatively misdiagnosed as adnexal masses (ovarian dermoid cyst, hemorrhagic corpus luteal cyst, or endometrioma), one case underwent salpingo-oophorectomy, two cases had abscess drainage with appendectomy, and two cases had abscess drainage with adhesiolysis [22]. The cases in the current study were followed up within a median duration of 47 months clinically and radiologically by doing contrast abdominal computed tomography, pelvic magnetic resonance imaging, and tumor markers. Follow-up was regular in the first year by imaging to make sure of the resolution of the inflammatory process, and response to medical treatment later follow-up imaging was done every year and according to the clinical manifestations. One case developed recurrent pelvic inflammatory disease (PID) after 62 months and was treated with conservative therapy with antibiotics. Ovarian cancer developed in another case after 80 months. This patient had laparoscopic drainage of pus from an adnexal mass and was followed up regularly; after 80 months, she developed a suspicious adnexal mass, mild ascites, and high cancer antigen (CA) 125 so she underwent optimal debulking; and the postoperative pathology was a malignant epithelial ovarian tumor. It was hypothesized that pelvic inflammatory disease (PID) and chronic inflammation stimulate the malignant transformation of ovarian epithelial cells [23]. Many epidemiological studies have demonstrated conflicting results about the incidence of ovarian cancer in pelvic inflammatory disease (PID) patients. It was reported 1.92 (95% CI, 1.27–2.92) as an adjusted hazard ratio of ovarian cancer in patients with pelvic inflammatory disease (PID) when compared to comparisons using a population-based design [24]. Other studies have correlated PID and the development of ovarian cancer especially among Asian women [25, 26]. In contrast, there are case–control studies that have excluded pelvic inflammatory disease (PID) as a potential risk of ovarian cancer development [27, 28]. The conflicts in these studies were due to a small sample size in some studies or a short follow-up duration in other studies. It could be explained also by the fact that pelvic inflammatory disease is an inflammatory disease that is not as long-lasting as endometriosis and is strongly related to the development of ovarian cancer [29].
Conclusion
Benign adnexal masses such as tubo-ovarian abscess secondary to pelvic inflammatory disease should be considered a differential diagnosis in patients with radiological suspicious adnexal masses when the tumor markers such as cancer antigen 125 are normal or mildly elevated, especially in premenopausal women, and the clinical presentations are not specific to either benign or malignant adnexal diseases. Laparoscopic assessment will be beneficial in the diagnosis of these cases with possible intraoperative frozen section to avoid extensive surgeries with its morbidities.
Data availability
The data generated and analyzed within this study are not publicly available and will be available with the corresponding author upon reasonable request.
References
Rajkotia K, Veeramani M, Macura KJ (2006) Magnetic resonance imaging of adnexal masses. Top Magn Reson Imaging 17(6):379–397
Nougaret S, Nikolovski I, Paroder V, Vargas HA, Sala E, Carrere S, Tetreau R, Hoeffel C, Forstner R, Lakhman Y (2019) MRI of tumors and tumor mimics in the female pelvis: anatomic pelvic space–based approach. Radiographics. 39(4):1205
Ozat M, Altinkaya SO, Gungor T, Çağlar M, Zergeroglu S, Karaca M, Besli M, Mollamahmutoglu L (2011) Extraovarian conditions mimicking ovarian cancer: a single center experience of 15 years. Arch Gynecol Obstet 284(3):713–719
Yamamoto Y, Yamada R, Oguri H, Maeda N, Fukaya T (2009) Comparison of four malignancy risk indices in the preoperative evaluation of patients with pelvic masses. Eur J Obstet Gynecol Reprod Biol 144(2):163–167
Van Holsbeke C, Van Calster B, Testa AC, Domali E, Lu C, Van Huffel S, Valentin L, Timmerman D (2009) Prospective internal validation of mathematical models to predict malignancy in adnexal masses: results from the international ovarian tumor analysis study. Clin Cancer Res 15(2):684–691
Campion EW, Brunham RC, Gottlieb SL, Paavonen J (2015) Pelvic inflammatory disease. N Engl J Med 372(21):2039–2048
Landers DV, Sweet RL (1983) Tubo-ovarian abscess: a contemporary approach to management. Rev Infect Dis 5(5):876–884
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Shetty M (2019) Nonovarian Mimics of Ovarian Malignancy. Semin Ultrasound CT MR 40(4):319–331
Kim MY, Rha SE, Oh SN, Jung SE, Lee YJ, Kim YS, Byun JY, Lee A, Kim MR (2009) MR imaging findings of hydrosalpinx: a comprehensive review. Radiographics. 29(2):495–507
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL (2018) American Cancer Society Cancer Facts & Figures 2018. American Cancer Society, Atlanta
Goodwin K, Fleming N, Dumont T (2013) Tubo-ovarian abscess in virginal adolescent females: a case report and review of the literature. J Pediatr Adolesc Gynecol 26(4):e99–e102
Kim YJ, Youm J, Kim JH, Jee BC (2014) Actinomyces-like organisms in cervical smears: the association with intrauterine device and pelvic inflammatory diseases. Obstet Gynecol Sci 57(5):393–396
Hamilton W, Peters TJ, Bankhead C, Sharp D (2009) Risk of ovarian cancer in women with symptoms in primary care: a population-based case-control study. BMJ 339
Ebell MH, Culp MB, Radke TJ (2016) A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med 50(3):384–394
Brunham RC, Gottlieb SL, Paavonen J (2015) Pelvic inflammatory disease. N Engl J Med 372(21):2039–2048
Wang T, Li W, Wu X, Yin B, Chu C, Ding M, Cui Y (2016) Tubo-ovarian abscess (with/without pseudotumor area) mimicking ovarian malignancy: role of diffusion-weighted MR imaging with apparent diffusion coefficient values. PLoS One 11(2):e0149318
Zhang Y, Lei H, Wang MY, Chen Y, Wang MY (2012) Pelvic tuberculosis mimicking ovarian carcinoma with adnexal mass and very high serum level of CA125. J Obstet Gynaecol 32(2):199–200
Sopik V, Rosen B, Giannakeas V, Narod SA (2015) Why have ovarian cancer mortality rates declined? Part III. Prospects for the future. Gynecol Oncol 138(3):757–761
Lareau SM, Beigi RH (2008) Pelvic inflammatory disease and tubo-ovarian abscess. Infect Dis Clin N Am 22(4):693–708
Rakheja R, Makis W, Hickeson M (2011) Bilateral tubo-ovarian abscess mimics ovarian cancer on MRI and 18 F-FDG PET/CT. Nucl Med Mol Imaging 45:223–228
Cho HW, Koo YJ, Min KJ, Hong JH, Lee JK (2017) Pelvic inflammatory disease in virgin women with tubo-ovarian abscess: a single-center experience and literature review. J Pediatr Adolesc Gynecol 30(2):203–208
Kisielewski R, Mazurek A, Laudański P, Tołwińska A (2013) Inflammation and ovarian cancer–current views. Ginekol Pol 84(4):293–297
Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, Wu SC, Lai YL (2011) Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol 12(9):900–904
Piao J, Lee EJ, Lee M (2020) Association between pelvic inflammatory disease and risk of ovarian cancer: an updated meta-analysis. Gynecol Oncol 157(2):542–548
Zhou Z, Zeng F, Yuan J, Tang J, Colditz GA, Tworoger SS, Trabert B, Su X (2017) Pelvic inflammatory disease and the risk of ovarian cancer: a meta-analysis. Cancer Causes Control 28:415–428
Parazzini F, La Vecchia C, Negri E, Moroni S, Dal Pino D, Fedele L (1996) Pelvic inflammatory disease and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev 5(8):667–669
Rasmussen CB, Kjaer SK, Albieri V, Bandera EV, Doherty JA, Høgdall E, Webb PM, Jordan SJ, Rossing MA, Wicklund KG, Goodman MT (2017) Pelvic inflammatory disease and the risk of ovarian cancer and borderline ovarian tumors: a pooled analysis of 13 case-control studies. Am J Epidemiol 185(1):8–20
Chene G, Ouellet V, Rahimi K, Barres V, Provencher D, Mes-Masson AM (2015) The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynecol Obstet 130(1):27–30
Acknowledgements
The authors are grateful to all their colleagues at the Oncology Center, Mansoura University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors have contributed to the study’s conception, design, material preparation, and data collection. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The Institutional Review Board (IRB) of the Faculty of Medicine, Mansoura University, approved this study with the code number R.23.04.2146. All the patients included in this study have read and approved the consent for surgical procedures.
Consent for Publication
Not Applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abouzid, A., Shetiwy, M., Hossam, A. et al. Pelvic Inflammatory Disease Mimicking Ovarian Cancer: A Case Series from A Tertiary Cancer Center. Indian J Surg (2024). https://doi.org/10.1007/s12262-023-04006-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-023-04006-5