Abstract
Embryological, anatomical, and immunological differences between the right-sided and left-sided colons are well known, but the difference in oncological behavior of colon tumors has only recently become the main subject of studies. Published articles propose that there is a difference not only in symptoms, but also in survival. Our aim was to analyze the clinicopathological and oncological differences among our patients who had been operated for colon cancer in our department. We examined the historical data of our patients who underwent colon resection for malignancy between 1st of January 2016 and 31st of December 2018. Tumor markers, histological results, postoperative complications, and oncological therapies were investigated. The primary outcome was overall survival. We analyzed our patients’ survival data with Kaplan–Meier log-rank test and Cox regression analysis. In our study, 267 patients were enrolled. One hundred thirty-three (49.8%) patients had right-sided colon cancer; 134 (50.2%) patients had left-sided colon cancer. Patients with right-sided colon cancer were significantly more likely to have mucinous adenocarcinoma (p = 0.037). No significant differences were revealed in overall survival between right-sided colon cancer and left-sided colon cancer patients (p = 0.381). Additional subgroup analysis showed that there were no significant differences in overall survival for laterality neither in the metastatic group (p = 0.824) nor in the non-metastatic group (p = 0.345). Based on the conflicting previous study results, our findings repeatedly highlight that the relationship between tumor location in the colon and overall survival is not straightforward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide [1]. In Hungary, CRC is the second most common cause of cancer-related death in both men and women [2]. Colon cancers can be divided into two groups based on the localization of the primary tumor. Right-sided colon cancer (RCC) and left-sided colon cancer (LCC) have different embryological, physiological, genetic, and clinical characteristics, which lead to differences in prognosis and in the outcome of disease [3]. Anatomically, the proximal colon arises from the midgut and receives its major blood supply through the superior mesenteric artery; the distal colon arises from the hindgut and is supplied by the inferior mesenteric artery. Clinically, patients with RCC are older and more likely to be women and are seen at first with more advanced and poorly differentiated tumor stages [4]. For these above mentioned reasons, numerous studies have reported that oncological outcomes of colon cancer are different according to the location of the tumor. In the literature, there are some controversial results regarding patients with non-metastatic colon cancer. Some studies have suggested no difference or better survival for right-sided tumor, but two meta-analyses showed that survival is worse for patients with right-sided colon cancer [5,6,7,8].
The effect of sidedness on determining the CRC prognosis still remains controversial. Our aim was to analyze the clinicopathological and oncological differences among our patients who had been operated for colon cancer.
Patients and Methods
Study Design
We examined the historical data of our patients of the Department of Surgery, Moritz Kaposi General Hospital, Kaposvár, Hungary, who underwent colon resection for malignancy between 1st of January 2016 and 31st of December 2018. We analyzed the data of 267 patients.
Inclusion and Exclusion Criteria
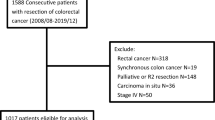
The inclusion criteria were as follows: (1) patients older than 18 years; (2) patients who had histopathologically confirmed colon cancer; and (3) radical surgical resection (resection margin-R0) was performed. The exclusion criteria were as follows: (1) patients who had unresectable tumor; (2) patients who had rectal cancer (when the primary tumor was aboral to 15 cm of anal verge); (3) patients who had undergone preoperative chemotherapy before surgery; (4) patients who had R1 or R2 resections; (5) patients lost to follow-up.
Patients’ Record Assessment and Follow-up
We analyzed carcinoembryonic antigen (CEA) levels from the preoperative routine blood test based on the international reference value. The resected colon tumors were histopathologically classified according to the seventh edition of the tumor-node-metastasis (TNM) classification. Besides the tumor markers and histological results, we also investigated the type of surgery (laparoscopic or open), postoperative complications, and oncological therapies. We attempted to identify unique clinicopathological characteristics of RCC and compare them with those of LCC. In the present study, rectosigmoid, descending colon, and splenic flexure tumors were considered LCC, whereas transverse colon, hepatic flexure, ascending colon, and cecal tumors were considered RCC. All evidence of recurrence was obtained from patient medical records. Patients were followed-up every 6 months.
Statistical Analysis
We used STATA software to perform all statistical analyses. Categorical variables were presented as a frequency or rate. Continuous variables were presented as the median with quartile range or the mean. For categorical data analysis, we used Pearson’s chi-squared test, which determines the statistically significant difference between the expected frequencies and the observed frequencies in more categories of a contingency table. The Kaplan–Meier estimate method with a log-rank test was used to accomplish univariate analysis. The hazard ratio (HR) and 95% confidence intervals (CI) estimated from the univariate and multivariate Cox regression analysis were detailed. P-value equal or less than 0.05 was considered statistically significant.
Ethics
Oral and written informed consent was obtained from the patients. The study was approved by the Ethics Review Committee of the Moritz Kaposi General Hospital.
Results
A total of 267 patients were enrolled in our study (Fig. 1). Baseline characteristics of patients comparing the two sides are presented in Table 1. One hundred thirty-three (49.8%) patients had RCC; 134 (50.2%) patients had LCC. We examined overall 149 (55.81%) men and 118 (44.19%) women, with a median age of 67.93 years (range 37–91). The median follow-up period was 29.13 months (range 0–49). During the follow-up period, 35 (13.1%) patients died.
Laparoscopic surgery was carried out in 48 patients (17.98%) and open procedure was performed in 219 cases (82.02%). In 56.18% of the patients, there were no lymph node metastases. No significant difference was determined in sex, age, clinical mode of presentation, and tumor size comparing the two sides. Patients with RCC were significantly more likely to have mucinous adenocarcinoma histology (p = 0.037).
With Kaplan–Meier log-rank test, no significant differences were revealed in overall survival (OS) between RCC and LCC patients (p = 0.381, Fig. 2). There were no significant differences in OS between the two sides in terms of age, sex, tumor size, N stage, M stage, CEA level, type of adenocarcinoma, number of reoperations, and postoperative complications. We investigated our patients’ data also altogether with the log-rank test and we found that T stage (p = 0.023), N stage (p < 0.001), M stage (p < 0.001), grade (p = 0.012), vascular (p < 0.001) and perineural (p < 0.001) invasion, elevated CEA level (p < 0.001), reoperation (p = 0.022), and postoperative complication (p < 0.001) had significant effect on OS (Fig. 3). Dukes’ stages were also significant (p < 0.001) variables with regard to the OS.
Kaplan–Meier curves for overall survival for the entire cohort. a T stage (p = 0.023), b N stage (p < 0.001), c M stage (p < 0.001), d grade (p = 0.012), e vascular invasion (p < 0.001), f perineural invasion (p < 0.001), g elevated CEA level (p < 0.001), h reoperation (p = 0.022), i postoperative complication (p < 0.001)
In univariate Cox regression analysis, the N stage (HR = 2.77, 95%CI = 1.38–5.56, p = 0.004), M stage (HR = 3.45, 95%CI = 1.77–6.75, p < 0.001), elevated CEA level (HR = 4.46, 95%CI = 1.68–11.85, p = 0.003), reoperation (HR = 3.17, 95%CI = 1.12–8.99, p = 0.030), and postoperative complication (HR = 3.26, 95%CI = 1.59–6.65, p = 0.0001) were significantly associated with worse OS. In multivariate Cox regression analysis, reoperation (HR = 4.10, 95%CI = 1.21–13.92, p = 0.024), elevated CEA level (HR = 2.88, 95%CI = 0.99–8.32, p = 0.050), and M stage (HR = 2.47, 95%CI = 0.99–6.15, p = 0.050) remained significant and were associated with worse OS (Table 2).
Patients were also analyzed based on their metastatic status. There were 216 (80.90%) patients in the non-metastatic group and 51 (19.10%) patients in the metastatic group. The additional subgroup analysis revealed that in the metastatic group, there were significantly more lymph node metastasis in case of LCC (p = 0.018) and had a significantly higher CEA level (p = 0.023). There was no strong evidence of interaction for other factors and no significant differences were found in OS for laterality neither in the metastatic group (p = 0.824), nor in the non-metastatic group (p = 0.345).
Discussion
In our cohort study, controversially to an article published in 2008, there were no significant differences in sex, age, and tumor size comparing the two sides [4]. However, similar to the result of a systematic review, we found significantly more mucinous adenocarcinomas in RCC [9]. Reviews and multiple studies have reported clinicopathological or genetic differences between RCC and LCC [9,10,11,12,13]. With regard to OS in our study, stratified analyses demonstrated that there was no significant difference between RCC versus LCC patients. Examining the entire cohort, regardless of laterality, we proved that preoperatively elevated CEA levels significantly reduced survival in patients with colon cancer. Adjuvant oncological treatment did not significantly affect survival. Significant difference was revealed in survival in terms of pathological T stage, grade, and perineural and vascular invasions. In case of lymph node positivity and distant metatasis, survival was significantly worse, and reoperation or postoperative complication also reduced survival. One previously published article also emphasized the importance of lymph node positivity for cancer-specific mortality regardless of tumor sidedness [14].
According to the data of 414 patients, the OS and disease-free survival (DFS) was found to be lower in RCC patients compared to LCC [15]. Similarly to our results, a study with 427 patients found no difference in OS comparing the right- and left-sided colorectal cancer, and there was a significantly higher OS among patients with low CEA values [16]. In a study published in 2020, the authors also did not observe differences in overall, disease-specific, or relative survival in patients with RCC versus LCC [17]. It was emphasized in a large sample–sized study that there was no survival difference between RCC and LCC in the following situations: older than 68 years old, T3–4, N0, poorly differentiated, and undifferentiated diseases [18].
Several authors have reported that metastatic RCC is associated with poorer long-term outcomes than LCC [19,20,21], whereas other authors have reported that non-metastatic RCC is associated with a better prognosis [6, 22]. In our additional subgroup analysis, we found that in the metastatic group, there were significantly more lymph node metastasis in case of LCC and these patients had a significantly higher CEA level. There was no significant difference in OS with regards to laterality. In a cohort of 53,801 patients, no overall difference was demonstrated in 5-year mortality rate between right- and left-sided colon cancer after adjusting for various variables [23]. It is notable that this investigation only included patients aged 65 and above, and their study was conducted over a longer period compared to ours. In a study including 6365 patients, laterality was also not associated with differences in long-term survival [5]. Based on a study that included 1725 patients, no significant difference was determined between the RCC and LCC groups in terms of survival, although the median survival time was higher in the LCC group (62 vs. 43 months) [24]. In an article published in 2016, the authors analyzed 91,416 patients with stage I to III colon cancer resected between 2004 and 2012. They found improved OS for patients with RCC, but sidedness was not associated with survival among patients with stage III, only in stage I and II disease [6].
The limitation of our study is the relatively small sample size and short follow-up, but we will continue the study based on our preliminary work. Results of previously published studies are not consistent; therefore, the extent to which disease sidedness is prognostic remains unclear. Further prospective studies are required.
Conclusion
Comparing right-sided colon cancer and left-sided colon cancer, we found significantly more mucinous adenocarcinoma histology type in right-sided colon cancer, but there was no significant difference in overall survival. Based on the conflicting previous study results, our findings repeatedly highlight that the relationship between tumor location in the colon and overall survival is not straightforward. It is important to plan further clinical trials to clarify the existence of the difference between the two sides of the colon for the purpose of individual patient management.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691. https://doi.org/10.1136/gutjnl-2015-310912
Boncz I, Brodszky V, Péntek M, Agoston I, Nagy Z, Kárpáti K, Kriszbacher I, Fuszek P, Gulácsi L (2010) The disease burden of colorectal cancer in Hungary. Eur J Health Econ 10(Suppl 1):S35-40. https://doi.org/10.1007/s10198-009-0192-z
Gervaz P, Bucher P, Morel P (2004) Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol 88(4):261–266. https://doi.org/10.1002/jso.20156
Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, Endo H, Shiratori Y (2008) Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol 23(3):418–423. https://doi.org/10.1111/j.1440-1746.2007.04923.x
Karim S, Brennan K, Nanji S, Berry SR, Booth CM (2017) Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol 3(10):1386–1392. https://doi.org/10.1001/jamaoncol.2017.1016
Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, Güller U (2016) Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 16(1):6–13. https://doi.org/10.1186/s12885-016-2412-0
Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y (2016) The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg 20(3):648–655. https://doi.org/10.1007/s11605-015-3026-6
Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G, Barni S (2017) Prognostic survival associated with left-sided vs right-sided colon cancer a systematic review and meta-analysis. JAMA Oncol 3(2):211–219. https://doi.org/10.1001/jamaoncol.2016.4227
Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK (2015) Is right-sided colon cancer different to left-sided colorectal cancer? - A systematic review. Eur J Surg Oncol 41(3):300–308. https://doi.org/10.1016/j.ejso.2014.11.001
Bustamante-Lopez LA, Nahas SC, Nahas CSR, Pinto RA, Marques CFS, Cecconello I (2019) Is there a difference between right- versus left-sided colon cancers? Does side make any difference in long-term follow-up? Arq Bras Cir Dig 32(4):e1479. https://doi.org/10.1590/0102-672020190001e1479
Cienfuegos JA, Baixauli J, Arredondo J, Pastor C, Martínez Ortega P, Zozaya G, Martí-Cruchaga P, Hernández Lizoáin JL (2018) Clinico-pathological and oncological differences between right and left-sided colon cancer (stages I-III): analysis of 950 cases. Rev Esp Enferm Dig 110(3):138–144. https://doi.org/10.17235/reed.2017.5034/2017
Ulanja MB, Rishi M, Beutler BD, Sharma M, Patterson DR, Gullapalli N, Ambika S (2019) Colon cancer sidedness, presentation, and survival at different stages. J Oncol 2019:4315032. https://doi.org/10.1155/2019/4315032
Fukata K, Yuasa N, Takeuchi E, Miyake H, Nagai H, Yoshioka Y, Miyata K (2020) Clinical and prognostic differences between surgically resected right-sided and left-sided colorectal cancer. Surg Today 50(3):267–274. https://doi.org/10.1007/s00595-019-01889-4
Lai HW, Wei JC, Hung HC, Lin CC (2019) Tumor sidedness influences prognostic impact of lymph node metastasis in colon cancer patients undergoing curative surgery. Sci Rep 9(1):19892. https://doi.org/10.1038/s41598-019-56512-w
Lim DR, Kuk JK, Kim T, Shin EJ (2017) Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: which side is better outcome? Medicine (Baltimore) 96(42):e8241. https://doi.org/10.1097/MD.0000000000008241
Odeny TA, Farha N, Hildebrandand H, Allen J, Vazquez W, Martinez M, Paluri RK, Kasi A (2020) Association between primary perioperative CEA ratio, tumor site, and overall survival in patients with colorectal cancer. J Clin Med 9(12):3848. https://doi.org/10.3390/jcm9123848
Klose J, Kloor M, Warschkow R, Antony P, Liesenfeld LF, Büchler MW, Schneider M, Tarantino I (2021) Does side really matter? Survival analysis among patients with right- versus left-sided colon cancer: a propensity score-adjusted analysis. Ann Surg Oncol 28(5):2768–2778. https://doi.org/10.1245/s10434-020-09116-y
Qiu MZ, Pan WT, Lin JZ, Wang ZX, Pan ZZ, Wang FH, Yang DJ, Xu RH (2018) Comparison of survival between right-sided and left-sided colon cancer in different situations. Cancer Med 7:1141–1150. https://doi.org/10.1002/cam4.1401
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, Townsend AR, Moore J, Roy A, Tomita Y, Karapetis C (2015) Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121(6):830–835. https://doi.org/10.1002/cncr.29129
Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ, Müller T, Hurwitz HI, Saltz L, Falcone A, Lenz HJ (2015) Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 107(3):427. https://doi.org/10.1093/jnci/dju427
Zhao B, Lopez NE, Eisenstein S, Schnickel GT, Sicklick JK, Ramamoorthy SL, Clary BM (2020) Synchronous metastatic colon cancer and the importance of primary tumor laterality - a National Cancer Database analysis of right- versus left-sided colon cancer. Am J Surg 220(2):408–414. https://doi.org/10.1016/j.amjsurg.2019.12.002
Moritani K, Hasegawa H, Okabayashi K, Ishii Y, Endo T, Kitagawa Y (2014) Difference in the recurrence rate between right- and left-sided colon cancer: a 17-year experience at single institution. Surg Today 44(9):1685–1691. https://doi.org/10.1007/s00595-013-0748-5
Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, Smith MA (2011) Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results—Medicare data. J Clin Oncol 29:4401–4409. https://doi.org/10.1200/JCO.2011.36.4414
Helvaci K, Eraslan E, Yildiz F, Tufan G, Demirci U, Berna Oksuzoglu O, Yalcintas Arslan U (2019) Comparison of clinicopathological and survival features of right and left colon cancers. J BUON 24(5):1845–1851
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception of the work, drafted the work, approved the final version to be published, and agreed to be accountable for all aspects of the work. In detail: AB, SK, and ZsK designed the study. AB, LT, and ECs collected the data. AB and ZsK made the statistical analysis. AB, AD, DT, MM, and ZsK wrote and reviewed the main manuscript text and prepared figures and tables.
Corresponding author
Ethics declarations
Ethics Approval
The study protocol was approved by the Ethics Review Committee of the Moritz Kaposi General Hospital.
Consent to Participate
Oral and written informed consent was obtained from all patients.
Consent for Publication
Not applicable (not a case report).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biró, A., Ternyik, L., Somodi, K. et al. Comparison of Resected Malignant Tumors of the Right- and Left-Sided Colon—Is There a Difference?. Indian J Surg 84, 971–978 (2022). https://doi.org/10.1007/s12262-021-03209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-021-03209-y