Abstract
We report an extremely rare case of primary aortocaval fistula with simultaneous development of an aortoenteric fistula in a 68-year-old man. The patient developed under oral anticoagulation a spontaneous intracaval aortic rupture. An emergency intervention was performed with a covering of the fistula with an aorto-uniiliac stent graft and a femoro-femoral crossover bypass. One week later, the patient was transferred to our institution with the diagnosis of a psoas abscess and a suspected concomitant aortoenteric fistula. We performed a complete explantation of the endograft and implanted it after extensive debridement an aortobiiliac bypass, made of bovine pericardium. The postoperative course was complicated, first by bleeding from the left iliac anastomosis, and then by bleeding from the proximal aortic anastomosis. The entire graft was explanted and an axillo-femoral bypass was implanted. The patient then developed a multi-organ failure and died 3 months later. If possible, an extended surgical debridement and resection of all infected tissue with in situ reconstruction is the gold standard. However, with this therapy, there is still a high risk of reinfection. Long-term antibiotic management is mandatory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aortocaval fistula (ACF) is an abnormal connection between the aorta and inferior vena cava and a rare and life-threatening condition. The most common causes are penetrating trauma and abdominal aortic aneurysm and the incidence is less than one percent [1]. Aortoenteric fistula (AEF) is likewise a rare and life-threatening condition. A contained ruptured of the infrarenal aorta associated with simultaneous primary aortoenteric fistula (PAEF) and primary aortocaval fistula (PACF) has not yet been reported in the literature.
Case Presentation
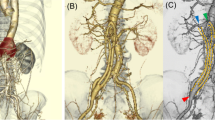
A 68-year-old man was admitted to the emergency department with an onset of abdominal and flank pain in the past week. The patient’s medical history included atrial fibrillation, arterial hypertension, chronic kidney disease, coronary artery disease with previous coronary stent implantation, and smoking. Also, a recent development of chronic heart insufficiency was diagnosed. Due to a suspected urinary tract infection, antibiotic treatment was initiated. Despite the antibiotic treatment, the symptoms were not relieved, and computed tomography (CT) was performed. A contained rupture of infrarenal aortic with high calcification of the wall and retroperitoneal hematoma without evidence of aortic aneurysm were shown. Previously, the patient had a phenprocoumon overdose, with an INR of 12 at admission. An emergency endovascular repair was performed. In addition to the aortic rupture, an aortocaval fistula was also detected during intraoperative angiography. An endovascular aorto-uni-iliac stent graft and a femoro-femoral crossover bypass were implanted (Fig. 1). The results of the blood cultures were negative for any bacteria. The patient was discharged on the 7th postoperative day from the hospital in good general condition and declining inflammatory markers. One week after discharge, the patient was found at home unconscious. After awakening, he complained of abdominal pain and had a fever accompanied by a heart rate of 240 bpm. A CT scan showed a left retroperitoneal abscess. An empiric broad-spectrum antibiotic therapy was started. Furthermore, CT-guided drainage of the abscess was performed. The results of blood cultures and pus culture showed Klebsiella pneumoniae, Enterococcus faecium, and Candida tropicalis. Antibiotic and antifungal management was accordingly instituted. The further clinical course was complicated by septic shock with cardiorespiratory insufficiency. The patient was then transferred to our institution in an ongoing state of sepsis. The patient was operated via a median laparotomy. Intraoperatively, aortoenteric fistula was located in Jejunum (10 cm distal to the duodenum). The fistula in the jejunum was resected and the defect was sutured in a single button technique. A complete explantation of the aorto-uni-iliacal endograft was carried out (Fig. 2). Reconstruction of the resected aorta was achieved by a surgeon-made bovine pericardium in situ aortobiiliac aortic graft (LeMaitre Vascular, Burlington, USA) (Fig. 3). The abscess was extensively debrided, rinsed, drained, and filled out with an omental flap. The omental flap also covered the new graft. The postoperative course was complicated, first by bleeding from the left iliac anastomosis, and then by bleeding from the proximal aortic anastomosis. The complete bypass was explanted and an axillo-femoral bypass was implanted. The patient then developed a multi-organ failure and died 3 months later.
Discussion
Aortocaval fistula represents a life-threatening condition. Without treatment, ACF has a very poor prognosis. Some authors have described a triad of signs associated with this pathology, which are cardiac failure, abdominal bruit, and lower extremity edema [2]. However, this classic triad is only present in 20–50% of patients [2]. Other findings associated with an ACF include hematuria, elevation of the jugular venous pressure, pulmonary edema, ascites, and pulsating varicose veins. However, our patient only had lower extremity edema associated with abdominal and flank pain. In cases of large fistula, an elevated preload rapidly leads to congestive heart failure resulting from left to right shunt. Decompensated congestive heart failure occurs in 35% of all ACF patients with a large shunt. In the present case, echocardiography showed a moderate dilatation of the right atrium and ventricle. Even though in case of emergency meticulous planning of the treatment is not feasible, in stable patients treated in an elective setting it is mandatory. This helps to avoid massive and “hard to control” bleeding from the inferior vena cava. The complexity of our case makes it difficult to compare to the literature, probably the first treatment with a stent graft was the best option to stabilize the patient. Endovascular treatment with EVAR may provide a quick solution a lower risk of 30-day mortality but is also associated with high complication rates. This should be seen as bridging therapy, since sequelae like endoleaks and, if an infection is present, reinfections are a significant problem. In the reported literature, such complications occur in 50% of ACF cases treated via endovascular means [3].
In the case of surgical AEF repair, there are various operative techniques. All of these involve the complete removal of all stent-graft components. Infrarenal aortic ligation and extra-anatomic bypass is usually chosen when the level of contamination is high [4]. However, high risks of graft occlusion resulting in amputation (up to 29%), aortic stump disruptions (up to 20%), and reinfection (up to 20%) have been reported [5]. An in situ reconstruction, with previous complete endograft removal and extensive debridement followed by omental flap, represents the other main surgical technique, and this was used, to avoid most of the risks of extra-anatomic technique [6]. Nevertheless, there is still a high risk of reinfection. In situ aortic reconstruction is using Dacron or polytetrafluoroethylene graft with omental coverage preferred in the case of PADF [7]. Schaefers et al. reported satisfactory outcomes with rifampicin-soaked polyester grafts [8]. Other authors prefer xenogenic or autogenic materials. In our case, the infected stent graft was removed and the ACF was smaller than 10 mm in diameter, bleeding was easy to control by digital compression, and closure of the ACF was achieved by direct suturing. The reconstruction of the resected aorta was achieved by a surgeon-made bovine pericardium in situ aortobiiliac aortic graft. Furthermore, bovine pericardium has a lower reinfection risk compared to nonbiological grafts. Even though there is a lack of guidelines, there is more consensus about antibiotic treatment. It should be selected based on the result of the sensitivity test administered for at least 6 weeks after surgery [8].
Conclusion
In conclusion, a timely diagnosis of primary aortoenteric fistula/aortocaval fistula is important to prevent lethal heart failure or GI bleeding. This is extremely important for planning the surgical strategy. Not unexpectedly, our patient fared poorly and eventually died of multi-organ failure after multiple reoperations.
Change history
14 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Brightwell RE, Pegna V, Boyne N (2013) Aortocaval fistula: current management strategies. ANZ J Surg 83:31–35
Maeda H, Umezawa H, Goshima M et al (2007) Surgery for ruptured abdominal aortic aneurysm with an aortocaval and iliac vein fistula. Surg Today 37:445–448
Orion KC, Beaulieu RJ, Black JH 3rd (2016) Aortocaval fistula: is endovascular repair the preferred solution? Ann Vasc Surg 31:221–228
Murphy EH, Szeto WY, Herdrich BJ, Jackson BM, Wang GJ, Bavaria JE et al (2013) The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg 58:1179–1185
Kuestner LM, Reilly LM, Jicha DL, Ehrenfeld WK, Goldstone J, Stoney RJ (1995) Secondary aortoenteric fistula: contemporary outcome with use of extraanatomic bypass and infected graft excision. Vasc Surg 21:184–195 (discussion: 195-6)
Kahlberg A, Rinaldi E, Piffaretti G, MAEFISTO collaborators et al (2016) Results from the multicenter study on aortoenteric fistulization after stent grafting of the abdominal aorta (MAEFISTO). J Vasc Surg 64(2):313–320
Ranasinghe W, Loa J, Allaf N, Guney D (2011) Primary aortoenteric fistulae: the challenges in diagnosis and review of treatment. Ann Vasc Surg 25:386
Schaefers JF, Donas KP, Panuccio G, Kasprzak B, Heine B, Torsello GB, Osada N (2019) Usai MV Outcomes of surgical explantation of infected aortic grafts after endovascular and open abdominal aneurysm repair. Eur J Vasc Endovasc Surg 57(1):130–136
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A., Marchiori, E., Oberhuber, A. et al. Fatal Case of a Contained Ruptured of the Infrarenal Aorta due to Simultaneous Primary Aortocaval Fistula and Aortoenteric Fistula. Indian J Surg 84, 856–859 (2022). https://doi.org/10.1007/s12262-021-03071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-021-03071-y