Abstract
Calprotectin is a positive acute-phase protein participating in innate immune responses and inflammatory processes. This protein is produced mainly in neutrophils, which infiltrate inflamed tissues and then increase the level of calprotectin in plasma, urine, or body secretions. Its measurement is used in the diagnosis of many inflammatory diseases of the gastrointestinal tract. Here, we reviewed the studies evaluating the utility of calprotectin when the patient is suspected of acute appendicitis, one of the most common causes of abdominal pain. Fecal and serum calprotectin provide clinicians additional information as compared to routinely performed laboratory analyses. Moreover, among all forms of the protein, the fecal calprotectin seems to be a particularly promising biomarker due to its high resistance to degradation in the stool. In the future, innovative methods in the form of neural networks may play a valuable role in developing such panels. These findings are important because current literature showed that sensitive and specific markers of acute appendicitis are still urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calprotectin (CP) is a small acidic protein belonging to the S100 family whose members comprise pseudo-EF-hands to coordinate Ca2 + ions. CP is a heterodimer consisting of MRP8/S100A8 and MRP14/S100A9 subunits containing iron, manganese, zinc, and calcium-binding sites [1]. CP is not only expressed mainly by neutrophils, but is also produced in monocytes and macrophages [2]. The metal-binding properties of CP lead to its antimicrobial functions in the nutritional immunity mechanism. The chelation of zinc and manganese reduces the microbial access to these valuable nutrient sources. On the other hand, recent observations provided novel insight into the microbicidal action of CP, which may be partly independent of metal bioavailability [3].
Extracellular CP plays a role in the innate immune system as one of the damage-associated molecular patterns (DAMPs), also called alarmins. These structures are recognized by receptors expressed on cells involved in nonspecific defense mechanisms, which initiate inflammation [4]. The role of CP in inflammation involves numerous mechanisms. It activates the NF-κB pathway, induces antinociceptive effect, inhibits immunoglobulin synthesis, and is a chemotactic factor for macrophages and neutrophils. What is more, the cell death-inducing activity of CP is also observed [5]. CP is one of the positive acute-phase proteins, so its concentration is increased in inflammatory sites. It is found not only within a cell but also in plasma, urine, and body secretions [6]. Upregulated CP appears in numerous pathological states, such as acute respiratory infections, rheumatoid arthritis, cystic fibrosis, multiple sclerosis, and inflammatory disorders affecting the gastrointestinal (GI) tract. The latter are characterized by an elevated level of CP especially in the stool. This fact becomes the subject of research on acute appendicitis (AA) diagnosis, in which CP may have a potential clinical application.
Increased Level of Fecal Calprotectin in Gastrointestinal Disorders
During the inflammation of the intestinal tissue, the mucosal layer becomes more permeable. Moreover, when the intestine is infected, bacterial components act as stimuli for the release from immune cells. These reasons may contribute to the migration of neutrophils and the following secretion of CP into the fecal matter, which is then named fecal calprotectin (FC) [7]. It is highly resistant to degradation by bacteria and pancreatic or intestinal proteases. In addition, CP is stable for at least a week at room temperature and can be easily quantified by enzyme-linked immunosorbent assay (ELISA) [8]. Moreover, the main advantage of FC is direct contact with the mucosa that allows it to detect intestinal inflammatory conditions far more precisely than biomarkers measured in serum [9]. Taking it into consideration, FC is an excellent noninvasive biomarker of intestinal inflammation in some GI diseases. Among the disorders affecting the GI tract, inflammatory bowel diseases (IBD) are of particular importance in the context of the FC level. Two predominant types of IBD (Crohn’s disease (CD) and ulcerative colitis (UC)) are characterized by chronic and relapsing intestinal inflammatory lessions [10]. The diagnosis of IBD involves biopsy and endoscopic examination; however, these procedures are unpleasant, have a risk of complications, and require a lot of commitment from medical staff. To limit the use of colonoscopy, non-invasive markers of intestinal inflammation were sought. One of them is FC with reliable clinical sensitivity and specificity [11]. The designation of its levels allows us to select patients who are more likely to need a colonoscopy and to distinguish IBD from functional diseases, such as irritable bowel syndrome (IBS). As a result of this approach, the number of colonoscopies performed in patients without IBD is minimized [12]. The percentage of false-negative FC is only about 6–8%. Moreover, it was shown that FC is an excellent tool to estimate endoscopic activity of IBD [13]. Therefore, FC seems to be a reliable marker for predicting mucosal healing and, on the other side, for relapse of IBD [14, 15]. FC is also overactive during the course of other GI diseases. Its higher concentrations are found in samples of colorectal cancer (CRC) and adenomas. Currently, the measurement of FC may be used as a screening for CRC. Unfortunately, the results of updating meta-analysis from 2018 do not recommend the use of FC as a lone screening tool [16]. However, FC seems to have application in diagnosing and monitoring of diverticulitis and microscopic colitis (MC) [17]. These numerous clinical applications have led to further hypotheses linking the use of FC in inflammatory disorders of the GI tract. Currently, it is subject to considerable debate whether FC levels help diagnose the most common cause of abdominal surgical intervention, acute appendicitis (AA).
Methods
Searching Strategy
A systematic literature search was carried out to identify all original studies that analyzed the role of both fecal and serum calprotectin in management of adult patients with AA.
The systematic literature search involved the following databases: OVID MEDLINE and EMBASE. The search query consisted of the combination of the following keywords: “fecal calprotectin,” “calprotectin,” “MRP8/14,” “S100A8/9,” “acute appendicitis,” “gastrointestinal.” Results were limited to relevant papers published in English in 2012–2021. The first search was performed on 2 February 2020, and the search was updated on 11 October 2020, with a final revision on 9 November 2020.
Study Selection and Risk of Bias
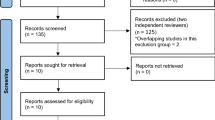
The references in all the included studies were reviewed for more eligible articles. Each article was reviewed independently by three researchers (AM, JF, MW) for inclusion according to the inclusion and exclusion criteria, which follow. Disagreements regarding article selection were resolved through discussion until consensus was reached or resolved by discussion between authors LD and MW. Case series studies on accrual/historical adult patient records were included. Conference abstracts were excluded. Articles were also excluded if they were not in English, or the studies were preclinical research or commentaries. A standardized form was used to extract data from the included studies. Extracted details were study population and demographics, details of interventions and controls, study methodology, and information to assess bias. Data extraction was performed independently by four authors, and discrepancies were resolved through discussion with the other co-authors (Fig. 1).
Outcome Assessment
The main outcome was the accuracy of both fecal and serum calprotectin in management of AA adult patients. In addition, eligible pediatric studies were referred to in “Discussion.”
Ethical Considerations
Ethics committee approval was not required for this study because it was a systematic review. Patient consent was not required because no patients or patient-identifiable data were involved in the study.
Results
Acute Appendicitis
Acute appendicitis is one of the predominant indications for emergency surgery, with an estimated lifetime incidence of 7%. Contrary to other etiologies of acute abdomen, AA occurs more commonly in children and young adults than in elderly patients [18]. Despite a high rate of incidence, the satisfying diagnostic approach has not been established yet. In AA, it is indispensable to have a diagnostic tool with high sensitivity to perform the surgical intervention early and then to prevent perforation. In addition, a high specificity is desirable to avoid unnecessary surgical intervention. To achieve this goal, clinical assessment, imaging strategies, and novel laboratory markers are used in daily clinical practice [19, 20]. The group of biomarkers includes white blood cell count (WBC), granulocytes, c-reactive protein (CRP), the proportion of polymorphonuclear cells, and the level of CP [21]. CP is overproduced during the course of AA as a consequence of high neutrophil recruitment to damaged mucosa of the appendix and thus has gained much interest in AA diagnosis [22].
Serum Calprotectin
What is worth noting is that the initial studies on the role of CP in AA were focused on the protein level in the serum (Fig. 2). They showed circulating form as a marker with high sensitivity but very low specificity rates (16–53, 6%) [23, 24]. Most findings suggested its poor discrimination in the form of the low area under the curve (AUC) values in receiver operating characteristic (ROC) curve analysis. Nevertheless, the high sensitivity of CP could be helpful for clinicians to avoid surgical intervention in case of false-positive diagnosis. In addition, the level of serum CP may be able to bring more information than WBC and CRP used in routine clinical practice, which is not strongly correlated. The results of already published studies do not provide consistent data on the correlation between CP and other biomarkers [25, 26]. Handoffs note, the circulating protein in a single test is indicated as an excellent tool to identify complicated AA with necrosis or perforation, which requires clinical intervention [26].
A summary of information concerning the use of serum calprotectin (CP) in the diagnosis of acute appendicitis (AA). The biomarker provides high sensitivity but low specificity in adult screening. Considering pediatric studies, the APPY1 biomarker panel including serum CP is effective to identify the patients at low risk for AA. Moreover, it is helpful to distinguish complicated from uncomplicated AA in non-adults
Fecal Calprotectin: Additional Opportunities?
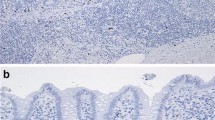
The hypothesis concerning FC as a tool for AA diagnosis was suggested in 2016 [27]. In the case series study, the post-appendectomy tissues were obtained from patients suspected of AA and then the expression of CP was measured by immunohistochemistry assay. The assessment of the immunostaining contained both epithelial and inflammatory cells of the vermiform appendix. These methods led to the finding that even if the appendicitis is uncomplicated, the immunochemical reaction with CP occurs. The main observation was the high overactivity of CP in the lumen of all inflamed samples which stays in contrast to the healthy appendix tissue. This may be the result of significant luminal infiltration of inflammatory cells.
The following pilot study tested the utility of FC as a marker of AA [28]. Stool samples were collected before the therapy and analyzed for FC concentration. The mean level of FC was higher in AA cases than that in controls (51.4 vs 24.8 µg/g), but these results were not significant. The AUC was 0.869 with the cutoff value of 51 µg/g, providing both sensitivity and specificity close to 80%. Importantly, FC was effective to distinguish AA from infectious enteritis (IE), which is another most common cause of pain located in the right lower quadrant. The IE activity of the neutrophils is much more intense and the FC significantly (p = 0.007) exceeds the levels observed in AA, with the mean value of 320.9 µg/g. A similar difference between IE and AA patients was not observed for routinely used WBC.
Recently, a study confirmed the significantly higher values of FC in the case of AA (240.5 vs 68.5 µg/g) [29]. Noteworthy is the high AUC of 0.928, which allows selecting the 106 µg/g cutoff point providing high sensitivity and specificity. Moreover, FC was significantly more active in complex (presence of a peri-appendicular abscess, gangrene, or perforation) than simple AA (206 vs. 304 µg/g). The studies on the role of FC in the diagnosis of AA have been showed in Table 1.
Discussion
The diagnosis of AA is currently undergoing an intense debate. The commonly used scoring systems result in common misdiagnosis and overtreatment. As a result, too many patients underwent unnecessary surgery and then have been exposed to severe complications such as mechanical bowel obstruction, an incisional hernia, or infertility. This explains why searching for effective AA biomarkers is so important [31].
Recent observations suggest that CP is a potential marker for AA diagnosis. However, a small number of studies on adults is the limitation. For this reason, it is worthy to focus on pediatrics. Considering several studies, the serum CP may serve a role in biomarker panel APPY1 diagnosing AA. The APPY1 panel including serum CP, WBC, and CRP has a high negative predictive value and allows reducing computer tomography utilization in patients suspected of suffering from AA, which is particularly important in case of non-adults [32,33,34,35]. More recently, a research evaluated the accuracy of clinical manifestations, laboratory analyses, and imaging methods for the diagnosis of AA [36]. They found that each marker used individually does not provide satisfying power, and the classic physical examination is still the most important single tool. The study provided new perspectives on the use of serum CP in diagnostic panels. The multivariate analysis of all markers was performed using neural networks. This innovative approach showed that the combination of physical examinations, biomarkers, including serum CP, and ultrasonography is a much more effective diagnostic panel than APPY1. This conclusion was in line with another study, in which a 6-part score including serum CP revealed great accuracy to predict appendicitis [37].
Another study offered new insight into CP utility due to the parallel measurement of the serum and fecal form in the same patients [30]. Comparing the cases with uncomplicated AA and controls, the FC was determined as a less accurate biomarker in diagnosis than serum CP (AUCFC = 0.669 vs AUCSERUM CP = 0.882). What is more, the comparison between patients with complicated AA and controls showed similar results (AUCFC = 0.951 vs AUCSERUM CP = 1.000). What is interesting is the cutoff levels of the serum CP indicated in ROC analysis have been observed on the relatively very low level as 670 ng/ml with maximal specificity. Considering current report literature, this value of serum CP may be higher in adult controls [38].
Conclusion
The level of fecal calprotectin is considered a valuable tool bringing additional data for clinicians in gastrointestinal disorders. Fecal calprotectin is a very stable form of calprotectin, which does not require a specific timing from the onset of symptoms to stool collection and further analysis. In Poland, fecal calprotectin tests are highly available due to the common use in inflammatory bowel diseases diagnosis. On the other hand, they are still relatively expensive. For now, its utility in acute appendicitis remains questionable. Unfortunately, several published studies are not consistent in their results of statistical parameters and accurate cutoff values and more reliable data are needed. However, in our opinion, it is particularly promising to focus on the fecal and serum calprotectin to develop in the future a more effective marker panel diagnosing acute appendicitis.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
F Shabani A Farasat M Mahdavi N Gheibi 2018 Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer Inflamm Res 67 801 812 https://doi.org/10.1007/s00011-018-1173-4
RM Ayling K Kok 2018 Fecal calprotectin Adv Clin Chem Elsevier Ltd 87 161 190 https://doi.org/10.1016/bs.acc.2018.07.005
Besold AN, Culbertson EM, Nam L, et al (2019) HHS public access. 10:1728–1742.https://doi.org/10.1039/c8mt00133b.Antimicrobial
JM Ehrchen C Sunderkötter D Foell 2009 The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer J Leukoc Biol 86 557 566 https://doi.org/10.1189/jlb.1008647
S Yui Y Nakatani M Mikami 2003 Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity Biol Pharm Bull 26 753 760 https://doi.org/10.1248/bpb.26.753
I Stríz I Trebichavský 2004 Calprotectin - a pleiotropic molecule in acute and chronic inflammation Physiol Res 53 245 253
WPNGW Pathirana SA Paul Chubb MJ Gillett SD Vasikaran 2018 Faecal calprotectin Clin Biochem Rev 39 77 90 https://doi.org/10.1097/mpg.0000000000001847
I Bjarnason 2017 The use of fecal calprotectin in inflammatory bowel disease Gastroenterol Hepatol (N Y) 13 53 56
E Burri C Beglinger 2014 The use of fecal calprotectin as a biomarker in gastrointestinal disease Expert Rev Gastroenterol Hepatol 8 197 210 https://doi.org/10.1586/17474124.2014.869476
M Sobczak A Fabisiak N Murawska 2014 Current overview of extrinsic and intrinsic factors in etiology and progression of inflammatory bowel diseases Pharmacol Reports 66 766 775 https://doi.org/10.1016/j.pharep.2014.04.005
AC Roon Von L Karamountzos S Purkayastha 2007 Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy Am J Gastroenterol 102 803 813 https://doi.org/10.1111/j.1572-0241.2007.01126.x
PF Rheenen Van E Vijver Van De V Fidler 2010 Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis BMJ 341 188 https://doi.org/10.1136/bmj.c3369
Rokkas T, Portincasa P, Koutroubakis IE (2018) Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis 27:299–306. https://doi.org/10.15403/jgld.2014.1121.273.pti
A Kostas SI Siakavellas C Kosmidis 2017 Fecal calprotectin measurement is a marker of shortterm clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease World J Gastroenterol 23 7387 7396 https://doi.org/10.3748/wjg.v23.i41.7387
R Mao Y Xiao X Gao 2012 Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies Inflamm Bowel Dis 18 1894 1899 https://doi.org/10.1002/ibd.22861
X Ye J Huai J Ding 2018 Diagnostic accuracy of fecal calprotectin for screening patients with colorectal cancer: a meta-analysis Turkish J Gastroenterol 29 397 405 https://doi.org/10.5152/tjg.2018.17606
Mari A, Baker FA, Mahamid M, et al (2019) Clinical utility of fecal calprotectin: potential applications beyond inflammatory bowel disease for the primary care physician. Ann Gastroenterol 32:425–430. https://doi.org/10.20524/aog.2019.0394
LK Gwynn 2001 The diagnosis of acute appendicitis: clinical assessment versus computed tomography evaluation J Emerg Med 21 119 123 https://doi.org/10.1016/s0736-4679(01)00353-5
B Stewart P Khanduri C McCord 2014 Global disease burden of conditions requiring emergency surgery Br J Surg 101 e9 22 https://doi.org/10.1002/bjs.9329
A Bhangu K Søreide S Saverio Di 2015 Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management Lancet (London, England) 386 1278 1287 https://doi.org/10.1016/S0140-6736(15)00275-5
DJ Shogilev N Duus SR Odom NI Shapiro 2014 Diagnosing appendicitis: evidence-based review of the diagnostic approach in 2014 West J Emerg Med 15 859 871 https://doi.org/10.5811/westjem.2014.9.21568
JJ Barcia N Reissenweber 2002 Neutrophil count in the normal appendix and early appendicitis: diagnostic index of real acute inflammation Ann Diagn Pathol 6 352 356 https://doi.org/10.1053/adpa.2002.36659
JF Bealer M Colgin 2010 S100A8/A9: a potential new diagnostic aid for acute appendicitis Acad Emerg Med 17 333 336 https://doi.org/10.1111/j.1553-2712.2010.00663.x
AM Mills DS Huckins H Kwok 2012 Diagnostic characteristics of S100A8/A9 in a multicenter study of patients with acute right lower quadrant abdominal pain Acad Emerg Med 19 48 55 https://doi.org/10.1111/j.1553-2712.2011.01259.x
G Thuijls JPM Derikx FJ Prakken 2011 A pilot study on potential new plasma markers for diagnosis of acute appendicitis Am J Emerg Med 29 256 260 https://doi.org/10.1016/j.ajem.2009.09.029
M Cikot KD Peker MA Bozkurt 2016 Plasma calprotectin level: usage in distinction of uncomplicated from complicated acute appendicitis World J Emerg Surg 11 7 12 https://doi.org/10.1186/s13017-016-0062-9
PC Ambe D Gödde L Bönicke 2016 Calprotectin could be a potential biomarker for acute appendicitis J Transl Med 14 107 https://doi.org/10.1186/s12967-016-0863-3
PC Ambe V Orth D Gödde H Zirngibl 2016 Improving the preoperative diagnostic accuracy of acute appendicitis. Can Fecal Calprotectin Be Helpful? PLoS One 11 e0168769 https://doi.org/10.1371/journal.pone.0168769
W Zhou H Qiao W Yuan 2019 Diagnostic utility of fecal calprotectin in patients presenting to the emergency department with suspected acute appendicitis Am J Emerg Med https://doi.org/10.1016/j.ajem.2019.10.022
SB Sarsu AB Erbagci H Ulusal 2017 The Place of calprotectin, lactoferrin, and high-mobility Group Box 1 protein on diagnosis of acute appendicitis with children Indian J Surg 79 131 136 https://doi.org/10.1007/s12262-015-1441-2
M Frountzas K Stergios D Kopsini 2018 Alvarado or RIPASA score for diagnosis of acute appendicitis? A meta-analysis of randomized trials Int J Surg 56 307 314 https://doi.org/10.1016/j.ijsu.2018.07.003
DS Huckins HK Simon K Copeland 2013 A novel biomarker panel to rule out acute appendicitis in pediatric patients with abdominal pain Am J Emerg Med 31 1368 1375 https://doi.org/10.1016/j.ajem.2013.06.016
J González Del Castillo FJ Ayuso V Trenchs 2016 Diagnostic accuracy of the APPY1 test in patients aged 2–20 years with suspected acute appendicitis presenting to emergency departments Emerg Med J 33 853 859 https://doi.org/10.1136/emermed-2015-205259
J Benito Y Acedo L Medrano 2016 Usefulness of new and traditional serum biomarkers in children with suspected appendicitis Am J Emerg Med 34 871 876 https://doi.org/10.1016/j.ajem.2016.02.011
H Depinet K Copeland J Gogain 2016 Addition of a biomarker panel to a clinical score to identify patients at low risk for appendicitis Am J Emerg Med 34 2266 2271 https://doi.org/10.1016/j.ajem.2016.08.018
Akgül F, Er A, Ulusoy E, et al (2019) Integration of physical examination, old and new biomarkers, and ultrasonography by using neural networks for pediatric appendicitis. Pediatr Emerg Care Publish Ah:1–7. https://doi.org/10.1097/pec.0000000000001904
J Benito S Fernandez M Gendive 2019 American Journal of Emergency Medicine A new clinical score to identify children at low risk for appendicitis ☆ Am J Emerg Med https://doi.org/10.1016/j.ajem.2019.05.050
M-A Meuwis G Vernier-Massouille JC Grimaud 2013 Serum calprotectin as a biomarker for Crohn’s disease J Crohns Colitis 7 e678 e683 https://doi.org/10.1016/j.crohns.2013.06.008
Funding
Funding supported by the Polish National Science Center (2016/23/N/NZ5/02564 to MW).
Author information
Authors and Affiliations
Contributions
Adam Makaro: drafting the work, the design of the work.
Łukasz Dziki: revising it critically for important intellectual content.
Jakub Fichna: revising it critically for important intellectual content.
Marcin Włodarczyk: the conception of the work. revising it critically for important intellectual content.
All the authors critically revised the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makaro, A., Dziki, Ł., Fichna, J. et al. On the Way to Improve Diagnostic Marker Panel for Acute Appendicitis in Adults: the Role of Calprotectin. Indian J Surg 84, 634–639 (2022). https://doi.org/10.1007/s12262-021-03063-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-021-03063-y