Abstract
Varicose vein is one type of venous insufficiency that presents with any dilated, elongated, or tortuous veins caused by permanent loss of its valvular efficiency. Destruction of venous valves in the axial veins results in venous hypertension, reflux, and total dilatation, causing varicosities and transudation of fluid into subcutaneous tissue. The first documented reference of varicose veins was found as illustrations on Ebers Papyrus dated 1550 B.C. in Athens. Evidence of surgical intervention was found in the 1860s. However dramatic advances of varicose vein management occurred in the latter half of twentieth century. Varicose veins affect from 40 to 60% of women and 15 to 30% men. Multiple intrinsic and extrinsic factors including age, gender, pregnancy, weight, height, race, diet, bowel habits, occupation, posture, previous DVT, genetics, and climate are considered to be the predisposing factors for formation of varicose vein. Other reported factors are hereditary, standing occupation, chair sitting, tight underclothes, raised toilet seats, lack of exercise, smoking, and oral contraceptives. Common symptoms are unsightly visible veins, pain, aching, swelling, itching, skin changes, ulceration, thrombophlebitis, and bleeding. The signs of varicose vein disease are edema, varicose eczema or thrombophlebitis, ulcers (typically found over the medial malleolus), hemosiderin skin staining, lipodermatosclerosis (tapering of legs above ankles, an “inverted champagne bottle” appearance), and atrophie blanche. Varicose vein is classified according to CEAP classification, the components of which are clinical, etiological, anatomy, and pathophysiology. The revised CEAP classification was published on 2020 based on four principles which were preservation of the reproducibility of CEAP, compatibility with prior versions, evidence-based medicine, and practicality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varicose veins constitute a progressive disease, and remission of the disease does not occur, except after pregnancy and delivery. During its course, the disease produces complication; most frequent are superficial thrombophlebitis, acute bleeding originating in one of the thin-walled varices, eczema, and, finally, skin ulceration [1].
History
The first documented reference of varicose veins was found as illustrations on Ebers Papyrus dated 1550 B.C. in Athens [2]. First patient who underwent operation for his varicose vein appears to be Canus Marius, the Roman tyrant. Greek philosopher Hippocrates (460–377 B.C.) described the use of compressive bandages and was advisor of small punctures in varicose veins. Aurelius Cornelius Celsus 25 B.C.–A.D.50) used linen bandages and plasters for leg ulcers. He treated them by exposure followed by avulsion with a blunt hook. Claudius Galen (A.D. 130–200) developed a method of bandaging which held the wound edges together. Galen’s theory of circulation remained standard theory for next 1400 years. William Harvey (1578–1657) proposed the theory of unidirectional blood circulation [3]. Giovanni Rima (1777–1843) introduced midthigh ligation of the saphenous vein.
The era of vascular intervention for varicose veins was modernized by Friedrich Trendelenburg, in the 1860s, who not only popularized his eponymous Trendelenburg test for saphenous reflux but also performed great saphenous vein (GSV) ligation by making a transverse upper thigh incision to ligate and divide the proximal GSV [1]. William Moore, an Australian surgeon, moved the site of ligation cephalad to the sapheno-femoral junction [1]. Ligation of the sapheno-femoral junction as it is practiced today was first described by John Homans in his paper in 1916 [1]. The Mayo Brothers, postulating that there would be additional benefit in removing the saphenous vein, pursued excision of the GSV through an incision extending from the groin to below the knee. This technique was initially improved by the use of an external “ring vein enucleator” [1]. The final technologic leap was introduction of the intraluminal stripper by Babcock [1]. The latter half of the twentieth century saw dramatic advances in diagnostic testing; however, surgical treatment of varicose veins benefited from only modest refinements after this innovation.
The twenty-first century has begun with a resurgence of interest and innovation in venous disease. Although sclerotherapy and endovenous thermal ablation occupy preeminent roles in the contemporary management of superficial venous disease, surgical approaches remain relevant when applied appropriately and executed expertly [4].
Epidemiology
It is generally agreed that varicose veins affect from 40 to 60% of women and 15 to 30% men [5]. In a study published on 1994, it was found that half of the adult population had minor stigmata of venous disease (women 50–55%; men 40–50%), but fewer than half of these will have visible varicose veins (women 20–25%; men 10–15%) [6]. However, more recently, large population studies such as Edinburgh Vein Study demonstrated an age-adjusted prevalence of truncal varices of 40% in men and 32% in women [7].
Definition
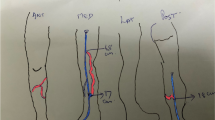
Varicose vein is one type of venous insufficiency which falls under the broad heading superficial venous disease [8]. In Western populations, the incidence of varicose veins varies with the definition applied. Most investigators favor the definition of Arnoldi, who said that varicosities are “any dilated, elongated, or tortuous veins, irrespective of size” [9] (Fig. 1).
The definition of Arnoldi is particularly useful because it presents a unifying concept for reticular varicosities, telangiectasias, and major varicose veins. Since all three are elongated, dilated, and have incompetent valves, they probably have a common origin and respond to the same physical forces and acquired influences [5]. The dilation and elongation implies that these abnormal veins have been responsive to effects of pressure. The dilation of a vein and valve annulus stretches beyond the capability of its leaflets to close together. Dodd and Cockett defined varicose veins, saying “a varicose vein is one which has permanently lost its valvular efficiency” [10] (Fig. 2). It was pressure over a course of time that causes a varix to become elongated, tortuous, pouched, and thickened.
Risk Factors
Among the theories that have been proposed to explain the cause of varicose veins is the hypothesis regarding weakness in the vein wall. Significantly reduced vein wall elasticity has suggested that the role of venous valves in development of varicose veins is secondary to changes in the elastic properties of the vein wall [11]. Estrogens, progestogens, or their associative action facilitate varicose vein development in individuals with factors which predispose them to vascular disorders (familial history, prolonged standing, obesity, and sedentary). They also aggravate the superficial venous state in these patients [12]. Wearing of tight undergarments produces proximal limb venous hypertension. A low-fiber diet predisposes to constipation and increased abdominal straining. Raised toilet seats prevent squatting during defecation. All these theories are related to venous hypertension, which itself is linked to development of venous insufficiency.
Common Predisposing Factors for Formation of Varicose Vein
Multiple intrinsic and extrinsic factors including age, gender, pregnancy, weight, height, race, diet, bowel habits, occupation, posture, previous DVT, genetics, and climate are the predisposing factors for formation of varicose vein [2]. Other factors documented in various studies are hereditary, standing occupation, chair sitting, tight underclothes, raised toilet seats, lack of exercise, smoking, and oral contraceptives.
Pathogenesis
Destruction of venous valves in the axial veins results in venous hypertension, reflux, and total dilatation, causing varicosities and transudation of fluid into subcutaneous tissue [2].
Development of Varicose Vein
All leg veins are equipped with valves at regular intervals. Together with the leg muscles and the pump function of the heart, these valves ensure that blood flows back to the heart against the force of gravity. Activating the leg muscles, for example by walking, compresses the deep veins lying between the muscles and forces the blood out of them. Healthy valves ensure that the blood flows in only one direction towards the heart and prevent any backflow to the feet. Most of the blood returns to the heart in the deep vein system. The superficial veins merely have a supporting role in blood transport, although they often develop into varicose veins. When superficial veins enlarge because of hereditary connective tissue weakness, the valves do not expand at the same time. This disrupts valve function, as the valves are no longer big enough to close the dilated vein (Fig. 2). As a result, there is a constant backflow to the feet that causes the vein to enlarge even further and varicose veins to develop (Fig. 3).
Saphena Varix
A saphena varix is a dilatation of the saphenous vein at the sapheno-femoral junction in the groin. As it displays a cough impulse, it is commonly mistaken for a femoral hernia; suspicion should be raised in any suspected femoral hernia if the patient has concurrent varicosities present in the rest of the limb. These can be best identified via duplex ultrasound and management is via high saphenous ligation.
Classifications
CEAP Classification—Creation
CEAP was suggested by John Porter in 1993 at the American Venous Forum. A consensus conference was held at the Sixth Annual Meeting of AVF in February, 1994. An international ad hoc committee chaired by Andrew Nicolaides with representatives from Australia, Europe, and the USA developed the first CEAP consensus document in 1994—“CEAP classification” [13]. It was accepted around the World by venous authorities of Europe, America and Asia. It was published in 11 languages in 5 continents. CEAP was updated in 1996 and revised in 2004 [13] (Table 1 and Table 2).
Since its introduction, CEAP has been demonstrated to be an excellent discriminative instrument and has become an accepted reporting standard for CVD research [14]. With time management of venous diseases has progressed, and many new modalities have been introduced which became popular in many fields. Over the years, criticisms of the instrument have included a lack of precise definitions resulting in a lack of reproducibility in assigning patients to specific clinical classes [15, 16]. In the 16 years since the last revision, an enhanced understanding of aspects of venous disease has identified gaps in the ability of CEAP to separately group patients with unique clinical attributes [17]. The necessity of further revision of CEAP was due with the advancement of phlebology. To address these advances, a taskforce was created for necessary revisions of CEAP classification. This task force comprised an international group of experts, as well as an advisory group of those who were involved in the creation and previous revision of the CEAP classification. Following a modified Delphi process, the task force adopted the following four “guiding principles”: preservation of the reproducibility of CEAP, compatibility with prior versions, evidence based medicine, and practicality. The revised CEAP remains a descriptive classification [18].
Changes in CEAP 2020
The CEAP 2020 taskforce adopted the following changes [19] (Table 3).
Clinical Domain
Revision in the “C” domain was done for the better understanding of the natural history between the subclasses. Corona phlebectatica appears to be a predictor of venous ulcer similar to other advanced skin changes and was placed as a subclass C4c in the class C4. The tendency of recurrence of varicose vein and venous ulcer was reflected by “r” in the revised CEAP. C2r indicates recurrent varicose vein, and C6r indicates recurrent venous ulcer.
Etiology Domain
Previously those patients who had no venous abnormality were classified as “En” (none). According to the modified CEAP, patients with clinical signs typically associated with venous disease will come under this subclass, if no other typical venous etiology is found. After the last revision of CEAP, the diverse of causality and development of newer treatment techniques raised the necessity to revise the secondary chronic venous disease (CVDs). To make it easily understandable, “Es” was separated into intravenous (Esi) and extravenous (Ese). The subclass “Esi” includes post-thrombotic changes, traumatic arteriovenous fistulas, primary intravenous sarcoma, or other luminal changes inside the vein. Unlike “Esi,” “Esc” does not reflect on conditions due to venous wall or valve damage, rather due to conditions affecting venous hemodynamics. It may be systemic (e.g., obesity and congestive heart failure) or locally by extrinsic compression (e.g., extravenous tumor and local perivenous fibrosis), or, at a distance, by muscle pump dysfunction due to motor disorders (paraplegia, arthritis, chronic immobility, and frozen ankle) [18].
Anatomy Domain
Previously 18 numerical designations were used to describe the venous segments of abdomen, pelvis, and lower extremities. Now it has been described by abbreviations which is more practical and easier for professional communication and publications. Anterior accessory saphenous vein was also included in the list of anatomical segments.
Pathophysiology Domain
The “P” component of CEAP was kept unchanged.
Venous Severity Scoring
The CEAP scoring was limited by several factors and was not popular. Rather it was found that severity scoring system based on CEAP was more desirable for research and daily practice. In 2000, the American Venous Forum (AVF), Ad Hoc Committee on Venous Outcomes Assessment, proposed the three-part Venous Severity Score: Venous Clinical Severity Score (VCSS), Venous Segmental Disease Score (VSDS), and Venous Disability Score (VDS)—a modification of the original CEAP disability score [20]. These scorings had been used to evaluate the severity of venous disease and to provide standardized evaluation of treatment effectiveness.
Venous Clinical Severity Score
The VCSS system includes 10 clinical descriptors (pain, varicose veins, venous edema, skin pigmentation, inflammation, induration, number of active ulcers, duration of active ulceration, size of ulcer, and compressive therapy use), scored from 0 to 3 (absent, mild, moderate, severe; total possible score, 30) that may be used to assess changes in response to therapy [21]. The revised VCSS score was published in 2010 and is currently being evaluated in studies for its validity and reliability.
Venous Segmental Disease Score
Venous Segmental Disease Score combines the anatomic and pathophysiologic components of CEAP. Major venous segments are graded according to presence of reflux and/or obstruction. It is entirely based on venous imaging, primarily duplex scan but also phlebographic findings. This scoring scheme weights 11 venous segments for their relative importance when involved with reflux and/or obstruction, with a maximum score of 10 [20].
Venous Disability Score
This modification to the original CEAP disability score substitutes prior normal activity level for the patient rather than ability to complete an 8-h workday.
Clinical Features
The common symptoms of varicose veins are unsightly visible veins, pain, aching, swelling (often worse on standing or at the end of the day), itching, skin changes, ulceration, thrombophlebitis, and bleeding. Edema, varicose eczema or thrombophlebitis, ulcers (typically found over the medial malleolus), hemosiderin skin staining, lipodermatosclerosis (tapering of legs above ankles, an “inverted champagne bottle” appearance), and atrophie blanche are common signs. Treatment should be considered when the patient is complaining of aching pain, leg heaviness, easy leg fatigue, superficial thrombophlebitis, external bleeding, ankle hyperpigmentation, lipodermatosclerosis, atrophie blanche, and venous leg ulcer.
Complications
Most common complications of varicose vein include aching pain, leg heaviness, and easy leg fatigue. Other complications are superficial thrombophlebitis, ankle hyperpigmentation, lipodermatosclerosis, atrophie blanche, and venous ulcer. Complications that require urgent management are superficial bleeding and superficial venous thrombosis. Rarely superficial venous thrombus may propagate to deep venous system.
References
Janugade HB, Patil BP, Tata NH, Saygaonkar HV, Janugade DH, Dokania V (2017) Clinical profile and management of lower limb varicose veins. J Evol Med Dent Sci 6(20):1615–1622. https://doi.org/10.14260/Jemds/2017/354
Iafrati MD, O’Donnell JRTF Varicose veins: surgical treatment. In: Cronenwett JL, Johnston KW (eds) Rutherford’s Vascular Surgery. Eighth edition, Chapter- 57, pp 869–884
Androutsos G, Karamanou M, Stefanadis C (2012) William Harvey (1578-1657): Discoverer of blood circulation. Hell J Cardiol 53:6–9
Kabnick LS, Sadek M (2014) Varicose veins: endovenous ablation and sclerotherapy. In: Cronenwett JL, Johnston KW (eds) Rutherford’s Vascular Surgery. Eighth edition, Chapter- 58, pp 885–901. Elsevier Saunders, Philadelphia, PA, USA
Bergan JJ (2004) Etiology and surgical management of varicose veins. In: Hobson, Wilson, Veith (eds) Vascular surgery: principles and practice. Third edition, revised and expanded. Chapter- 67, pp 949–962. Marcel Dekker Inc., New York, NY, USA
Callam MJ (1994) Epidemiology of varicose veins. Br J Surg 81(2):167–173
Evans CJ, Fowkes FG, Ruckley CV, Lee AJ (1999) Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health 53(3):149–153
Ghosh SK (2020) Trends on management of superficial venous disease. Int Surg J 7(8):2820–2823
Arnoldi CC (1957) The aetiology of primary varicose veins. Dan Med Bull 4:102–107
Borschberg E 1967 The prevalence of varicose veins in the lower extremities. Thesis. S Karger, Basel
Clarke GH, Vasdekis SN, Hobbs JT, Nicolaides AN (1992) Venous wall function in the pathogenesis of varicose veins. Surgery 111(4):402–408
Vin F, Allaert FA, Levardon M (1992) Influence of estrogens and progesterone on the venous system of the lower limbs in women. J Dermatol Surg Oncol 18(10):888–892
Bo E, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL et al (2004) Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 40:1248–1252
Allegra C, Antignani PL, Bergan JJ, Carpentier PH, Coleridge-Smith P, Cornu-Thenard A et al (2003) The “C” of CEAP: suggested definitions and refinements: an International Union of Phlebology conference of experts. J Vasc Surg 37:129–131
Cornu-Thenard A, Uhl JF, Carpentier PH (2004) Do we need a better classification than CEAP? Acta Chir Belg 104:276–282
Antignani PL, Cornu-Thenard A, Allegra C, Carpentier PH, Partsch H, Uhl JF (2004) Results of a questionnaire regarding improvement of ‘C’ in the CEAP classification. Eur J Vasc Endovasc Surg 28:177–181
Khilnani NM, Davies AH (2020) CEAP- a review of the 2020 revision. Phlebology 35(10):745–748
Lurie F The 2020 update of the CEAP classification: what is new? Eur J Vasc Endovasc Surg. https://doi.org/10.1016/j.ejvs.2020.04.020
Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H et al (2020) The 2020 update of the CEAP classification system and reporting standard. J Vasc Surg Venous Lymphat Disord 8(3):342–352 Available online 27 February 2020
Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL (2000) Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg 31:1307–1312
Passman MA, McLafferty RB, Lentz MF, Schneider JR, Lohr JM, Caprini JA et al (2011) Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum, National Venous Screening Program. Clinical research study from the American venous forum. J Vasc Surg 54(6):2s–9s
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghosh, S.K., Al Mamun, A. & Majumder, A. Clinical Presentation of Varicose Veins. Indian J Surg 85 (Suppl 1), 7–14 (2023). https://doi.org/10.1007/s12262-021-02946-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-021-02946-4