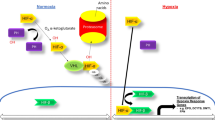
Abstract
Hypoxia is one of the main pathogenetic factors in liver, an organ that exhibits lower oxygen pressure than that in other organs. Liver hepatocytes have an approximately 10-fold higher oxygen demand than other cells for homeostatic metabolism. Moreover, several clinical studies have indicated that liver diseases might aggravate some kidney diseases. Therefore, it is critical to develop in vitro models for investigating diseases resulting from the interaction of liver and kidney under hypoxia. In this study, transwell plates were used to evaluate the interaction between proximal tubular epithelial cells and hepatocytes under hypoxia, which was induced using a mixture of sodium sulfite and cobalt chloride. Increased relative lactate dehydrogenase release was not observed in single cultures of proximal tubular epithelial cells and hepatocytes under hypoxic conditions. Compared to single cultures of proximal tubular epithelial cells and hepatocytes, relative lactate dehydrogenase releases were 1.70 and 1.50 times higher in co-cultures of proximal tubular epithelial cells and hepatocytes, respectively. Gene expressions of several markers such as serine palmitoyltransferase light chain 1 and ceramide synthetase 2 were analyzed to examine lipotoxicity under hypoxia. In addition, treatment with myriocin, a well-known ceramide inhibitor, reduced cytotoxicity in proximal tubular epithelial cells. The results of this study suggest that hypoxia might not be cytotoxic in separate monolayer cultures of proximal epithelial cells and hepatocytes. However, it might be cytotoxic due to interactions between these cell types, possibly due to an accumulation of ceramide, a result that can be described as lipotoxicity.
Similar content being viewed by others
References
Kusminski, C. M., S. Shetty, L. Orci, R. H. Unger, and P. E. Scherer (2009) Diabetes and apoptosis: lipotoxicity. Apoptosis., 14: 1484–1495.
Jun, S. Y. and H. S. Shin (2021) Analysis and prediction of surface condition of artificial skin based on CNN and ConvLSTM. Biotechnol. Bioprocess Eng., 26: 369–374.
Mukherjee, S., K. R. Aseer, and J. W. Yun (2020) Roles of macrophage colony stimulating factor in white and brown adipocytes. Biotechnol. Bioprocess Eng., 25: 29–38.
Goh, S., D. Kim, M. H. Choi, H. J. Shin, and S. Kwon (2019) Effects of bamboo stem extracts on adipogenic differentiation and lipid metabolism regulating genes. Biotechnol. Bioprocess Eng., 24: 454–463.
Chang, S. Y., E. J. Weber, V. S. Sidorenko, A. Chapron, C. K. Yeung, C. Gao, Q. Mao, D. Shen, J. Wang, T. A. Rosenquist, K. G. Dickman, T. Neumann, A. P. Grollman, E. J. Kelly, J. Himmelfarb, and D. L. Eaton (2017) Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight., 2: e95978.
Cho, H., D. Kim, and K. Kim (2018) Engineered co-culture strategies using stem cells for facilitated chondrogenic differentiation and cartilage repair. Biotechnol. Bioprocess Eng., 23: 261–270.
Theobald, J., A. Ghanem, P. Wallisch, A. A. Banaeiyan, M. A. Andrade-Navarro, K. Taškova, M. Haltmeier, A. Kurtz, H. Becker, S. Reuter, R. Mrowka, X. Cheng, and S. Wölfl (2018) Liver-kidney-on-chip to study toxicity of drug metabolites. ACS Biomater. Sci. Eng., 4: 78–89.
Lau, Y. Y., Y. H. Chen, T. T. Liu, C. Li, X. Cui, R. E. White, and K. C. Cheng (2004) Evaluation of a novel in vitro Caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metab. Dispos., 32: 937–942.
Li, C., T. Liu, X. Cui, A. S. Uss, and K. C. Cheng (2007) Development of in vitro pharmacokinetic screens using Caco-2, human hepatocyte, and Caco-2/human hepatocyte hybrid systems for the prediction of oral bioavailability in humans. J. Biomol. Screen., 12: 1084–1091.
Li, A. P., C. Bode, and Y. Sakai (2004) A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem. Biol. Interact., 150: 129–136.
Hamden, K., B. Jaouadi, S. Carreau, S. Bejar, and A. Elfeki (2010) Inhibitory effect of fenugreek galactomannan on digestive enzymes related to diabetes, hyperlipidemia, and liver-kidney dysfunctions. Biotechnol. Bioprocess Eng., 15: 407–413.
Palatini, P. and S. De Martin (2016) Pharmacokinetic drug interactions in liver disease: An update. World J. Gastroenterol., 22: 1260–1278.
Bale, S. S., L. Moore, M. Yarmush, and R. Jindal (2016) Emerging in vitro liver technologies for drug metabolism and inter-organ interactions. Tissue Eng. Part B Rev., 22: 383–394.
Suzuki, T., S. Shinjo, T. Arai, M. Kanai, and N. Goda (2014) Hypoxia and fatty liver. World J. Gastroenterol., 20: 15087–15097.
Byrne, C. D. (2009) Hypoxia and non-alcoholic fatty liver disease. Clin. Sci., 118: 397–400.
Jungermann, K. and T. Kietzmann (2000) Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology., 31: 255–260.
Kietzmann, T. (2017) Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol., 11: 622–630.
Lee, J. W., J. Ko, C. Ju, and H. K. Eltzschig (2019) Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med., 51: 1–13.
Nath, B. and G. Szabo (2012) Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology., 55: 622–633.
Balis, U. J., K. Behnia, B. Dwarakanath, S. N. Bhatia, S. J. Sullivan, M. L. Yarmush, and M. Toner (1999) Oxygen consumption characteristics of porcine hepatocytes. Metab. Eng., 1: 49–62.
Lee, J. S. and S. W. Cho (2012) Liver tissue engineering: Recent advances in the development of a bio-artificial liver. Biotechnol. Bioprocess Eng., 17: 427–438.
Aboelsoud, M. M., A. I. Javaid, M. O. Al-Qadi, and J. H. Lewis (2017) Hypoxic hepatitis - its biochemical profile, causes and risk factors of mortality in critically-ill patients: a cohort study of 565 patients. J. Crit. Care., 41: 9–15.
Waseem, N. and P. H. Chen (2016) Hypoxic hepatitis: a review and clinical update. J. Clin. Transl. Hepatol., 4: 263–268.
Paternostro, C., E. David, E. Novo, and M. Parola (2010) Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J. Gastroenterol., 16: 281–288.
Chalasani, N., Z. Younossi, J. E. Lavine, A. M. Diehl, E. M. Brunt, K. Cusi, M. Charlton, and A. J. Sanyal (2012) The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology., 55: 2005–2023.
Musso, G., R. Gambino, J. H. Tabibian, M. Ekstedt, S. Kechagias, M. Hamaguchi, R. Hultcrantz, H. Hagström, S. K. Yoon, P. Charatcharoenwitthaya, J. George, F. Barrera, S. Hafliðadóttir, E. S. Björnsson, M. J. Armstrong, L. J. Hopkins, X. Gao, S. Francque, A. Verrijken, Y. Yilmaz, K. D. Lindor, M. Charlton, R. Haring, M. M. Lerch, R. Rettig, H. Völzke, S. Ryu, G. Li, L. L. Wong, M. Machado, H. Cortez-Pinto, K. Yasui, and M. Cassader (2014) Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med., 11: e1001680.
Jiang, B., C. Ren, Y. Li, Y. Lu, W. Li, Y. Wu, Y. Gao, P. J. Ratcliffe, H. Liu, and C. Zhang (2011) Sodium sulfite is a potential hypoxia inducer that mimics hypoxic stress in Caenorhabditis elegans. J. Biol. Inorg. Chem., 16: 267–274.
Abudara, V., R. G. Jiang, and C. Eyzaguirre (2002) Behavior of junction channels between rat glomus cells during normoxia and hypoxia. J. Neurophysiol., 88: 639–649.
Klimova, T. and N. Chandel (2008) Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ., 15: 660–666.
Ghaly, A. E. and R. Kok (1988) The effect of sodium sulfite and cobalt chloride on the oxygen transfer coefficient. Appl. Biochem. Biotechnol., 19: 259–270.
Al Okail, M. S. (2010) Cobalt chloride, a chemical inducer of hypoxia-inducible factor-1a in U251 human glioblastoma cell line. J. Saudi Chem. Soc., 14: 197–201.
Kim, S. M., D. H. Kim, and D. J. Oh (2020) Development and evaluation of cell culture devices with the gas-permeable membrane. Biotechnol. Bioprocess Eng., 25: 62–70.
Kraus, N. A., F. Ehebauer, B. Zapp, B. Rudolphi, B. J. Kraus, and D. Kraus (2016) Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte., 5: 351–358.
Livak, K. J. and T. D. Schmittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods., 25: 402–408.
Lindner, K., U. Uhlig, and S. Uhlig (2005) Ceramide alters endothelial cell permeability by a nonapoptotic mechanism. Br. J. Pharmacol., 145: 132–140.
Chaurasia, B. and S. A. Summers (2015) Ceramides-lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab., 26: 538–550.
Kurek, K., P. Wiesiolek-Kurek, D. M. Piotrowska, B. Lukaszuk, A. Chabowski, and M. Zendzian-Piotrowska (2014) Inhibition of ceramide de novo synthesis with myriocin affects lipid metabolism in the liver of rats with streptozotocin-induced type 1 diabetes. Biomed Res. Int., 2014: 980815.
Yang, R. X., Q. Pan, X. L. Liu, D. Zhou, F. Z. Xin, Z. H. Zhao, R. N. Zhang, J. Zeng, L. Qiao, C. X. Hu, G. W. Xu, and J. G. Fan (2019) Therapeutic effect and autophagy regulation of myriocin in nonalcoholic steatohepatitis. Lipids Health Dis., 18: 179.
Qiu, X., X. Zhou, Y. Miao, and B. Li (2018) An in vitro method for nephrotoxicity evaluation using HK-2 human kidney epithelial cells combined with biomarkers of nephrotoxicity. Toxicol. Res., 7: 1205–1213.
Tanaka, T., M. Narazaki, and T. Kishimoto (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol., 6: a016295.
Sivertsson, L., M. Ek, M. Darnell, I. Edebert, M. Ingelman-Sundberg, and E. P. A. Neve (2010) CYP3A4 catalytic activity is induced in confluent Huh7 hepatoma cells. Drug Metab. Dispos., 38: 995–1002.
Mylonis, I., G. Simos, and E. Paraskeva (2019) Hypoxia-inducible factors and the regulation of lipid metabolism. Cells., 8: 214.
Zhang, T., C. Suo, C. Zheng, and H. Zhang (2019) Hypoxia and metabolism in metastasis. Adv. Exp. Med. Biol., 1136: 87–95.
Watt, M. J. and G. R. Steinberg (2008) Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem. J., 414: 313–325.
Liu, L., X. Shi, C. S. Choi, G. I. Shulman, K. Klaus, K. S. Nair, G. J. Schwartz, Y. Zhang, I. J. Goldberg, and Y. H. Yu (2009) Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes., 58: 2516–2524.
Timmers, S., J. de Vogel-van den Bosch, M. K. C. Hesselink, D. van Beurden, G. Schaart, M. J. Ferraz, M. Losen, P. Martinez-Martinez, M. H. De Baets, J. M. F. G. Aerts, and P. Schrauwen} (2011) Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS One., 6: e14503.
Bosma, M., S. Kersten, M. K. C. Hesselink, and P. Schrauwen (2012) Re-evaluating lipotoxic triggers in skeletal muscle: relating intramyocellular lipid metabolism to insulin sensitivity. Prog. Lipid Res., 51: 36–49.
Thomas, L. W. and M. Ashcroft (2019) Exploring the molecular interface between hypoxia-inducible factor signalling and mitochondria. Cell. Mol. Life Sci., 76: 1759–1777.
Semenza, G. L. (2007) Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J., 405: 1–9.
DeBerardinis, R. J. and N. S. Chandel (2020) We need to talk about the Warburg effect. Nat. Metab., 2: 127–129.
Brownsey, R. W., A. N. Boone, J. E. Elliott, J. E. Kulpa, and W. M. Lee (2006) Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans., 34: 223–227.
Taxiarchis, A., H. Mahdessian, A. Silveira, R. M. Fisher, and F. M. Van't Hooft (2019) PNPLA2 influences secretion of triglyceriderich lipoproteins by human hepatoma cells. J. Lipid Res., 60: 1069–1077.
Neuschwander-Tetri, B. A. (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology., 52: 774–788.
Chhabra, R. and M. Nanjundan (2020) Lysophosphatidic acid reverses Temsirolimus-induced changes in lipid droplets and mitochondrial networks in renal cancer cells. PLoS One., 15: e0233887.
Jin, J., Z. Lu, Y. Li, L. A. Cowart, M. F. Lopes-Virella, and Y. Huang (2018) Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLoS One., 13: e0193343.
Amemiya, F., S. Maekawa, Y. Itakura, A. Kanayama, A. Matsui, S. Takano, T. Yamaguchi, J. Itakura, T. Kitamura, T. Inoue, M. Sakamoto, K. Yamauchi, S. Okada, A. Yamashita, N. Sakamoto, M. Itoh, and N. Enomoto (2008) Targeting lipid metabolism in the treatment of hepatitis C virus infection. J. Infect. Dis., 197: 361–370.
Liu, X., D. E. Cooper, A. A. Cluntun, M. O. Warmoes, S. Zhao, M. A. Reid, J. Liu, P. J. Lund, M. Lopes, B. A. Garcia, K. E. Wellen, D. G. Kirsch, and J. W. Locasale (2018) Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell., 175: 502–513. e13.
Fan, J., J. J. Kamphorst, J. D. Rabinowitz, and T. Shlomi (2013) Fatty acid labeling from glutamine in hypoxia can be explained by isotope exchange without net reductive isocitrate dehydrogenase (IDH) flux. J. Biol. Chem., 288: 31363–31369.
Senkal, C. E., M. F. Salama, A. J. Snider, J. J. Allopenna, N. A. Rana, A. Koller, Y. A. Hannun, and L. M. Obeid (2017) Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab., 25: 686–697.
Ruiz-Argüello, M. B., G. Basáñez, F. M. Goñi, and A. Alonso (1996) Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J. Biol. Chem., 271: 26616–26621.
Montes, L. R., M. B. Ruiz-Argüello, F. M. Goñi, and A. Alonso (2002) Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 277: 11788–11794.
Acknowledgements
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2020R1A4A1016793), a National Research Foundation of Korea (NRF-2020R1F1A1048494), and an Inha University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Park, J., Heo, Y.J. & Kwon, S. Interaction Between Hepatocytes and Proximal Tubular Epithelial Cells in Hypoxia-induced Lipotoxicity. Biotechnol Bioproc E 27, 30–39 (2022). https://doi.org/10.1007/s12257-021-0137-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0137-7