Abstract
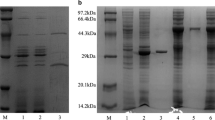
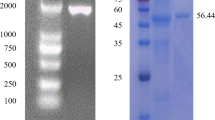
Microbial cellulases have become the mainstream biocatalysts due to their complex nature and widespread industrial applications. Here, homogeneous endoglucanase GluCB31 from Bacillus subtilis subsp. inaquosorum CBS31 was studied. GluCB31 was purified to 17.68-fold with an 8.33% yield and a specific activity of 1066.37 U/mg. Biochemical properties of GluCB31 were performed and the results are as follows; molecular mass of 35 kDa with an optimum pH at 7.5 and temperature at 50°C. GluCB31 was immobilized in calcium alginate gel and it exhibited the highest activity at 10°C higher temperature than soluble enzyme, as the entrapment in alginate gel made GluCB31 more stable. Kinetic studies showed the Vmax of 1293.33 ± 2.51 U/mg and Km of 0.0183 mg/mL. Enzymatic activity was activated by Tween-20 (106.7%), Tween-80 (111.6%), Triton X-100 (142.3%), SDS (135.5%), Mg++ (185.7%), Cu++ (167.6%), Zn++ (153.7%), Mn++ (106.3%), Ba++ (181.9%), Ni++ (107.2%) while inhibited by Fe++ (15.8%), β-mercaptoethanol (46.8%), EDTA (54.5%). Enthalpy, free energy, and entropy of activation were calculated to be 38.526 kJmol-1, 44.187 kJmol-1, and -17.518 Jmol-1K-1 respectively. Also, ΔGE-S and ΔGE-T were found to be -10.75 kJmol-1 and -45.92 kJmol-1 respectively. A low ΔS, ΔGE-S, and ΔGE-T values were signified enzyme-catalyzed reaction occurs at a fast rate and the existence of the enzyme in its stable state. Cellobiose was the major end product of hydrolysis. These attributes of GluCB31 demonstrated the diversity of catalytic activities and serve in various biotechnological processes, thus deserve to be developed as a bio-industrial agent.
Similar content being viewed by others
References
Nishida, Y., K. Suzuki, Y. Kumagai, H. Tanaka, A. Inoue, and T. Ojima (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochem. 89: 1002–1011.
Bhat, M. K. and S. Bhat (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 15: 583–620.
Lynd, L. R., P. J. Weimer, W. H. van Zyl, and I. S. Pretorius (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66: 506–577.
Pérez, J., J. Munoz-Dorado, T. de la Rubia, and J. Martinez (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 5: 53–63.
Robson, L. M. and G. H. Chambliss (1989) Cellulases of bacterial origin. Enzyme Microb. Technol. 11: 626–644.
Kotchoni, S. O., E. W. Gachomo, B. O. Omafuvbe, and O. O. Shonukan (2006) Purification and biochemical characterization of carboxymethyl cellulase (CMCase) from a catabolite repression insensitive mutant of Bacillus pumilus. Int. J. Agric. Biol. 8: 286–292.
Singh, V. K. and A. Kumar (1998) Production and purification of an extracellular cellulase from Bacillus brevis vs-1. IUBMB Life. 45: 443–452.
Lee, Y. J., B. K. Kim, B. H. Lee, K. I. Jo, N. K. Lee, C. H. Chung, Y. C. Lee, and J. W. Lee (2008) Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour. Technol. 99: 378–386.
Yin, L. J., H. H. Lin, and Z. R. Xiao (2010) Purification and characterization of a cellulase from Bacillus subtilis YJ1. J. Mar. Sci. Technol. 18: 466–471.
Yan, H., Y. Dai, Y. Zhang, L. Yan, and D. Liu (2011) Purification and characterization of an endo-1, 4-β-glucanase from Bacillus cereus. Afr J. Biotechnol. 10: 16277–16285.
Aygan, A., L. Karcioglu, and B. Arikan (2011) Alkaline thermostable and halophilic endoglucanase from Bacillus licheniformis C108. Afr. J. Biotechnol. 10: 789–796.
Regmi, S., Y. H. Choi, Y. S. Choi, M. R. Kim, and J. C. Yoo (2017) Antimicrobial peptide isolated from Bacillus amyloliquefaciens K14 revitalizes its use in combinatorial drug therapy. Folia Microbiol. 62: 127–138.
Regmi, S., Y. S. Choi, Y. H. Choi, Y. K. Kim, S. S. Cho, J. C. Yoo, and J. W. Suh (2017) Antimicrobial peptide from Bacillus subtilis CSB138: characterization, killing kinetics, and synergistic potency. Int. Microbiol. 20: 43–53.
Regmi, S., G. C. Pradeep, Y. H. Choi, Y. S. Choi, J. E. Choi, S. S. Cho, and J. C. Yoo (2016) A multi-tolerant low molecular weight mannanase from Bacillus sp. CSB39 and its compatibility as an industrial biocatalyst. Enzyme Microb. Technol. 92: 76–85.
Regmi, S., H. Y. Yoo, Y. H. Choi, Y. S. Choi, J. C. Yoo, and S. W. Kim (2017) Prospects for bio-industrial application of an extremely alkaline mannanase from Bacillus subtilis subsp. inaquosorum CSB31. Biotechnol. J. 12: 1700113
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Eyring, H. and A. E. Stearn (1939) The application of the theory of absolute reaction rates to proteins. Chem. Rev. 24: 253–270.
Dixon, M. and E. C. Webb (1979) Enzymes. 3rd ed., pp. 138–163. Academic Press, New York, USA.
Heinzelman, P., R. Komor, A. Kanaan, P. Romero, X. Yu, S. Mohler, C. Snow, and F. Arnold (2010) Efficient screening of fungal cellobiohydrolase class I enzymes for thermostabilizing sequence blocks by SCHEMA structure-guided recombination. Protein Eng. Des. Sel. 23: 871–880.
Gübitz, G. M., S. D. Mansfield, D. Böhm, and J. N. Saddler (1998) Effect of endoglucanases and hemicellulases in magnetic and flotation deinking of xerographic and laser-printed papers. J. Biotechnol. 65: 209–215.
Oksanen, T., J. Pere, L. Paavilainen, J. Buchert, and L. Viikari (2000) Treatment of recycled kraft pulps with Trichoderma reesei hemicellulases and cellulases. J. Biotechnol. 78: 39–48.
Ding, S., W. Ge, and J. A. Buswell (2002) Secretion, purification and characterisation of a recombinant Volvariella volvacea endoglucanase expressed in the yeast Pichia pastoris. Enzyme Microb. Technol. 31: 621–626.
Sudan, R. and B. K. Bajaj (2007) Production and biochemical characterization of xylanase from an alkalitolerant novel species Aspergillus niveus RS2. World J. Microbiol. Biotechnol. 23: 491–500.
Mawadza, C., R. Hatti-Kaul, R. Zvauya, and B. Mattiasson (2000) Purification and characterization of cellulases produced by two Bacillus strains. J. Biotechnol. 83: 177–187.
Bischoff, K. M., A. P. Rooney, X. L. Li, S. Liu, and S. R. Hughes (2006) Purification and characterization of a family 5 endoglucanase from a moderately thermophilic strain of Bacillus licheniformis. Biotechnol. Lett. 28: 1761–1765.
Lucas, R., A. Robles, M. T. García, G. Alvarez De Cienfuegos, and A. Gálvez (2001) Production, purification, and properties of an endoglucanase produced by the hyphomycete Chalara (Syn. Thielaviopsis) paradoxa CH32. J. Agric. Food Chem. 49: 79–85.
Andriani, D., C. Sunwoo, H. W. Ryu, B. Prasetya, and D. H. Park (2012) Immobilization of cellulase from newly isolated strain Bacillus subtilis TD6 using calcium alginate as a support material. Bioprocess Biosyst. Eng. 35: 29–33.
Busto, M. D., N. Ortega, and M. Perez-Mateos (1998) Characterization of microbial endo-β-glucanase immobilized in alginate beads. Acta Biotechnol. 18: 189–200.
Wang, F., R. X. Su, W. Qi, M. J. Zhang, and Z. M. He (2010) Preparation and activity of bubbling-immobilized cellobiase within chitosan-alginate composite. Prep. Biochem. Biotechnol. 40: 57–64.
Singh, J., N. Batra, and R. C. Sobti (2004) Purification and characterisation of alkaline cellulase produced by a novel isolate, Bacillus sphaericus JS1. J. Ind. Microbiol. Biotechnol. 31: 51–56.
de Marco, J. L. and C. R. Felix (2007) Purification and characterization of a beta-Glucanase produced by Trichoderma harzianum showing biocontrol potential. Braz. Arch. Biol. Technol. 50: 21–29.
Kim, J. Y., S. H. Hur, and J. H. Hong (2005) Purification and characterization of an alkaline cellulase from a newly isolated alkalophilic Bacillus sp. HSH-810. Biotechnol. Lett. 27: 313–316.
Madern, D., C. Ebel, and G. Zaccai (2000) Halophilic adaptation of enzymes. Extremophiles. 4: 91–98.
Wejse, P. L., K. Ingvorsen, and K. K. Mortensen (2003) Xylanase production by a novel halophilic bacterium increased 20-fold by response surface methodology. Enzyme Microb. Technol. 32: 721–727.
Tong, C. C., A. L. Cole, and M. G. Shepherd (1980) Purification and properties of the cellulases from the thermophilic fungus Thermoascus aurantiacus. Biochem. J. 191: 83–94.
Daniel, R. M. (1996) The upper limits of enzyme thermal stability. Enzyme Microb. Technol. 19: 74–79.
Nosoh, Y. and T. Sekiguchi (1990) Protein engineering for thermostability. Trends Biotechnol. 8: 16–20.
Zale, S. E. and A. M. Klibanov (1986) Why does ribonuclease irreversibly inactivate at high temperatures? Biochemistry. 25: 5432–5444.
Vieille, C. and J. G. Zeikus (1996) Thermozymes: identifying molecular determinants of protein structural and functional stability. Trends Biotechnol. 14: 183–190.
D’Amico, S., J. C. Marx, C. Gerday, and G. Feller (2003) Activity-stability relationships in extremophilic enzymes. J. Biol. Chem. 278: 7891–7896.
Amiri, H., K. Karimi, and H. Zilouei (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 152: 450–456.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01319101 and PJ01331001)” Rural Development Administration, Republic of Korea and National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2018R1D1A1B05050137).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest The authors declare that further, they have no conflict of interest.
Ethical Statement This article does not comprise any studies through human participants or animals performed by any of the authors.
Informed Consent Here, Informed consent was attained from all individual participants included in the study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Regmi, S., Choi, Y.S., Kim, Y.K. et al. Endoglucanase Produced by Bacillus subtilis Strain CBS31: Biochemical Characterization, Thermodynamic Study, Enzymatic Hydrolysis, and Bio-industrial Applications. Biotechnol Bioproc E 25, 104–116 (2020). https://doi.org/10.1007/s12257-019-0338-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0338-5