Abstract
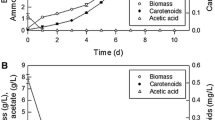
Use of photosynthetic bacteria to produce carotenoids has increased considerably in recent decades; however, few studies have been conducted to identify additional carotenoid producers. In this study, Rhodopseudomonas faecalis PA2 was shown to be capable of carotenoid production, and factors influencing this production were identified. The maximum carotenoid content was observed at an initial pH of 7 in the presence of 0.8% malic acid, 0.4% yeast extract, and 0.05% Fe3+ under light at 4,000 lux. Fe3+ significantly enhanced carotenogenesis, resulting in a production rate of 2.5 mg/g/day and a reduction in time to maximum production from 12 to 8 days. The carotenoid content and carotenoid yield under modified conditions were 413 mg/L and 13 mg/g (mg total carotenoids per gram of dry cells), respectively, representing an increase of 117% relative to the original condition. The biomass and carotenoid productivity reached 4 g/L/day and 51.6 mg/L/day, respectively. To the best of our knowledge, this is the first study of carotenoid production by Rps. faecalis PA2. The results indicated that the productivity of this organism under the aforementioned conditions was comparable to that of previously described purple photosynthetic bacterial species.
Similar content being viewed by others
References
Asker, D. and Y. Ohta (1999) Production of canthaxanthin by extremely halophilic bacteria. J. Biosci. Bioeng. 88: 617–621.
Khaneja, R., L. Perez-Fons, S. Fakhry, L. Baccigalupi, S. Steiger, E. To, G. Sandmann, T. C. Dong, E. Ricca, P. D. Fraser, and S. M. Cutting (2010) Carotenoids found in Bacillus. J. Appl. Microbiol. 108: 1889–1902.
Mata-Gomez, L. C., J. C. Montanez, A. Mendez-Zavala, and C. N. Aquilar (2014) Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell. Fact. 13: 1–11.
Darvin, M. E., W. Sterry, J. Lademann, and T. Vergou (2011) The role of carotenoids in human skin. Molecules 16: 10491–10506.
Tanaka, T., M. Shnimizu, and H. Moriwaki (2012) Cancer chemoprevention by carotenoids. Molecules 17: 3202–3242.
Kumar, S. R., M. Hosokawa, and K. Miyashita (2013) Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 11: 5130–5147.
Shegokar, R. and K. Mitri (2012) Carotenoid lutein: A promising candidate for pharmaceutical and nutraceutical applications. J. Diet. Suppl. 9: 183–210.
Brown, A. C., H. M. Leonard, K. J. McGraw, and E. D. Clotfelter (2013) Maternal effects of carotenoid supplementation in an ornamented cichlid fish. Funct. Ecol. 1–5.
Aksu, Z. and A. Eren Tugba (2005) Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Proc. Biochem. 40: 2985–2991.
Hu, Z. C., Y. G. Zheng, Z. Wang, and Y. C. Shen (2007) Production of astaxanthin by Xanthophyllomonas dendrorhous ZJUT46 with fed-batch fermentation in 2.0 m3 fermentor. Food Technol. Biotechnol. 45: 209–212.
Gu Z., D. Chen, Y. Han, Z. Chen, and F. Gu (2008) Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT 41: 1082–1088.
Takaichi, S. (2011) Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 9: 1101–1118.
Takaichi, S. (1999) Carotenoids and carotenogenesis in anoxygenic photosynthetic bacteria. pp. 39–69. In: H. A. Frank, A. J. Young, G. Britton, and R. J. Cogdell (eds.). The Photochemistry of Carotenoids. Kluwer Academic Publisher, Dordrecht, The Netherlands.
Wang, G., H. Grammel, K. Abou-Aisha, R. Sagesser, and R. Ghosh (2012) High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 78: 7205–7215.
Noparatnaraporn, N. and S. Nagai (1986) Selection of Rhodobactor sphaeroides P47 as useful source of single cell protein. J. Gen. Appl. Microbiol. 32: 351–359.
Chen, D., Y. Han, and Z. Gu (2006) Application of statistical methodology of the optimization of fermentative medium for carotenoids production by Rhodobacter sphaeroides. Proc. Biochem. 41: 1773–1778.
Kuo, F. H., Y. H. Chien, and C. J. Chen (2012) Effects of light sources on growth and carotenoid content of photosynthetic bacteria Rhodopseudomonas palustris. Bioresour. Technol. 113: 315–318.
Zhang, D., H. Yang, Z. Huang, W. Zhang, and S. J. Liu (2002) Rhodopseudomonas faecalis sp. nov., a phototrophic bacterium isolated from an anaerobic reactor that digests chicken faeces. Int. J. Syst. Evol. Microbiol. 52: 2055–2060.
Liu, B., G. Xie, W. Guo, J. Ding, and N. Ren (2011) Optimization of Photo-Hydrogen Production by Immobilized Rhodopseudomonas faecalis RLD-53. Nat. Resour. 2: 1–7. doi: 10.4236/nr.2011.21001.
Xie, G., B. Liu, D. Xing, J. Nan, J. Ding, H. Ren, W. Guo, and N. Ren (2012) Photo-hydrogen production by Rhodopseudomonas faecalis RLD-53 immobilized on the surface of modified activated carbon fibers. RSC Adv. 2: 2225–2228.
Hong, H. Y., B. Liu, J. Ding, J. Nan, G. Xie, L. Zhao, M. Chen, and N. Ren (2012) Enhanced photo-hydrogen production of Rhodopseudomonas faecalis RLD-53 by EDTA addition. Int. J. Hydrogen Energy 37: 8277–8281.
Noparatnaraporn, N., K. Sasaki, Y. Nishizawa, and S. Nagai (1986) Stimulation of vitamin B12 formation in aerobicallygrown Rhodopseudomonas gelatinosa under microaerobic condition. Biotechnol. Lett. 8: 491–496.
Hirayama, O. (1968) Lipids and lipoprotein complex in photosynthetic tissue: 4 lipid and pigments of photosynthetic bacteria. Agric. Biol. Chem. 32: 34–41.
An, G., J. Bielich, R. Auerbach, and E. A. Johnson (1991) Isolation and characterization of carotenoid hyperproducing mutants of yeast by flow cytometry and cell sorting. Nat. Biotechnol. 9: 70–73.
Latha, B. V., K. Jeevaratnam, H. S. Murali, and K. S. Manja (2005) Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DER-PDY from natural source. Indian J. Biotechnol. 4: 353–357.
Ainon, H., C. J. Tan, and S. Vikineswary. (2006) Biological Characterization of Rhodomicrobium vannielii isolated from a hot spring at Gadek, Malacca, Malaysia. Malays. J. Microbiol. 2: 15–21.
Cheirsilp, B. and S. Torpee (2012) Enhaced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 110: 510–516.
Hosseini Tafreshi, A. and M. Shariati (2009) Dunaliella biotechnology: methods and applications. J. Appl. Microbiol. 107: 14–35.
Kobayashi, M. K., N. M. Toshihide, and S. Nagai (1992) Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 74: 61–63.
Bhosale, P. and R. V. Gadre (2002) Manipulation of temperature and illumination conditions for enhanced ß-carotene production by mutant 32 of Rhodotorula glutinis. Lett. Appl. Microbiol. 34: 349–353.
Alcantara, S. and S. Sanchez (1999) Influence of carbon and nitrogen sources on Flavobacterium growth and zeaxanthin biosynthesis. J. Ind. Microbiol. Biotechnol. 23: 697–700.
Bhosale, P., A. J. Larson, and P. S. Bernstein (2004) Factorial analysis of tricarboxylic acid cycle intermediates for optimization of zeaxanthin production from Flavobacterium multivorum. J. Appl. Microbiol. 96: 623–629.
Higuchi, M. and G. Kikuchi (1963) Synthesis of bacteriochlorophyll by Rhodopseudomonas spheroids under dark-aerobic conditions. Nature 200: 1191–1192.
Castenholz, R. M. W. (1973) The possible photosynthetic use of sulfide by the filamentous phototrophic bacteria of hot springs. Limnol. Oceanogr. 18: 863–876.
Goodwin, T. W. (1980) The Biochemistry of Carotenoids. Chapman and Hall, London, UK.
Bhosale, P. (2004) Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 63: 351–361.
Tjahjono, A. E., Y. Hayama, T. Kakizono, Y. Terada, N. Nishio, and S. Nagai (1994) Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol. Lett. 16: 133–138.
Buzzini, P., A. Martini, M. Gaetani, B. Turchetti, U. M. Pagnoni, and P. Davoli (2005) Optimization of carotenoid production by Rhodotorula glutinis DBVPG7021 as a function of trace element concentration by means of response surface analysis. Enz. Microb. Technol. 36: 687–692.
Komemushi, S., H. Sakaki, H. Yokohama, and T. Fujita (1994) Effect of barium and other metals on the growth of a D-lactic acid assimilating yeast Rhodotorula glutinis N21. J. Antibact. Antifung. Agt. 22: 583–587.
Zhou, Q., P. Zhang, and G. Zhang (2014) Biomass and carotenoid production in photosynthetic bacteria wastewater treatment: Effects of light intensity. Bioresour. Technol. 171: 330–335.
Goksan, T., Y. Dumaz, and S. Gokpinar (2003) Effect of light paths lengths and initial culture density on the cultivation of Chaetoceros muelleri (Lemmermann, 1898). Aquaculture 217: 431–436.
Jalal, K. C. A., Z. A. Zaima, A. Zira, Z. Nor Hafizah, M. M. Rahman, B. Y. Kamaruzzaman, and H. N. Noor Faizul (2014) Carotenoid contents in anoxygenic phototrophic purple bacteria, Marichromatium sp. and Rhodopseudomonas sp. of tropical aquatic environment. Malay. Orient. J. Chem. 30: 607–613.
Getha, K., S. Vikineswary, and V. C. Chong (1998) Isolation and growth of the phototrophic bacterium Rhodopseudomonas palustris strain B1 in sago-starch-processing wastewater. World J. Microbiol. Biotechnol. 14: 505–511.
Prasertsan, P., W. Choorit, and S. Suwanno (1993) Optimization for growth of Rhodocyclus gelatinosus in seafood processing effluents. World J. Microbiol. Biotechnol. 9: 593–596.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saejung, C., Apaiwong, P. Enhancement of carotenoid production in the new carotenoid-producing photosynthetic bacterium Rhodopseudomonas faecalis PA2. Biotechnol Bioproc E 20, 701–707 (2015). https://doi.org/10.1007/s12257-015-0015-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0015-2