Abstract
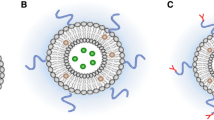
The preparation of fused materials using liposomes has been examined for several decades as a tool for the stabilization of heterogeneous enzymes. We investigated the liposomal encapsulation of lysosomal enzymes extracted from Saccharomyces cerevisiae. Liposomes were formed with L-α-phosphatidylcholine from egg yolk and cholesterol. To encapsulate whole lysosomal enzymes in liposomes made with and without cholesterol, L-α-phosphatidylcholine and cholesterol were added to chloroform at a ratio of 10:0 (L-α-phosphatidylcholine:cholesterol) and then evaporated for 10 min at 4°C. The residue after evaporation was mixed with lysosomal enzymes at the same ratio and then vortexed for 1 min and sonicated for 5 sec to encapsulate the enzymes. Liposome-encapsulated lysosomal enzymes were created using various amounts of lysosomal enzymes and cholesterol. The results indicated that the optimal encapsulation conditions were lipid:cholesterol ratios of 7:3 and 8:2. Liposome formation was confirmed by TEM imaging. After 1 day, two types of liposomes released small amounts of lysosomal enzymes. However, after 6 days, liposomes formed from mixtures of lipid and cholesterol did not exhibit any changes, whereas liposomes formed from only lipids released high amounts of lysosomal enzymes. Lysosomal enzymes encapsulated in liposomes have potential as important drug delivery carriers, as liposomes are able to control drug release and bioavailability.
Similar content being viewed by others
References
Bai, Y., H. Yang, W. Yang, Y. Li, and C. Sun (2007) Gold nanoparticles-mesoporous silica composite used as an enzyme immobilization matrix for amperometric glucose biosensor construction. Sens. Actuator B-Chem. 124: 179–186.
Azamian, B. R., J. J. Davis, K. S. Coleman, C. B. Bagshaw, and M. L. H. Freen (2002) Bioelectrochemical single-walled carbon nanotubes. J. Am. Chem. Soc. 124: 12664–12665.
Kim, B. C., S. Nair, J. Kim, J. Kim, J. H. Kwak, J. W. Grate, S. H. Kim, and M. B. Gu (2005) Preparation of biocatalytic nanofibres with high activity and stability via enzyme aggregate coating on polymer nanofibres. Nanotechnol. 16: 382–388.
Liao, M. H. and D. H. Chen (2001) Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnol. Lett. 23: 1723–1727.
Sun, H. S., X. Zhu, L. Zhang, X. Gu, J. Wang, J. Li, and Y. Zhang (2013) PEDA-coated magnetic nanoparticles for gene delivery to Hep G2 cells. Biotechnol. Bioproc. Eng. 18: 648–654.
Kim, J., J. W. Grate, and P. Wang (2006) Nanostructures for enzyme stabilization. Chem. Eng. Sci. 61: 1017–1026.
Klinger, A., D. Steinberg, D. Kohavi, and M. N. Sela (1998) Mechanism of adsorption of human albumin to titanium in vitro. J. Biomed. Mater. Res. Part A 36: 387–392.
Cheng, Y.M., X. H. Jin, D. G, H. F. Xia, and J. H. Chen (2013) Thermodynamics and kinetics of lysozyme adsorption onto two kinds of weak cation exchangers. Biotechnol. Bioproc. Eng. 18: 950–955.
Sokalingam, S. B. Madan, G. Raghunathan, and S. G. Lee (2013) In silico study on the effect of surface lysines and arginines on the electrostatic interactions and proteins stability. Biotechnol. Bioproc. Eng. 18: 18–26.
Wiesner, M. R., G. V. Lowry, P. Alvarez, D. Dionysiou, and P. Biswas (2006) Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 40: 4336–4345.
Coleman, S. E., I. V. Rijn, and A. S. Bleiveis (1970) Lysis of grouped and ungrouped Streptococci by lysozyme. Infect. Immun. 2: 563–569.
Han, S. and Y. Yang (2005) Antimicrobial activity of wool fabric treated with curcumin. Dyes Pigment. 64: 157–161.
Yoon, J., J. M. Park, S. K. Jung, K. Y. Kim, Y. H. Kim, and J. Min (2009) Characterization of antimicrobial activity of the lysosomes isolated from Saccharomyces cerevisiae. Curr. Microbiol. 59: 48–52.
Yoon, J., J. M. Park, K. J. Kim, Y. H. Kim, and J. Min (2009) Antimicrobial activity of the cell organelles, lysosomes, isolated from egg white. J. Microbiol. Biotechnol. 11: 1364–1368.
Goyal, M, K. I. Roy, U. C. Banerjee, V. K. Sharma, and A. K. Bansal (2009) Role of benzyl alcohol in the prevention of heatinduced aggregation and inactivation of hen egg white lysozyme. Eur. J. Pharm. Biopharm. 71: 367–376.
Ibrahim, H. R., T. Matsuzaki, and T. Aoki (2001) Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 506: 27–32.
Guérin-Dubiard, C., M. Pasco, A. Hietanen, A. Quiros del Bosque, F. Nau, and T. Croguennec (2005) Hen egg white fractionation by ion-exchange chromatography. J. Chromatogr. A. 1090: 58–67.
Gregoriadis, G. and D. E. Neerunjun (1974) Control of the rate of hepatic uptake and catabolism of liposome-entrapped protein injected into rats. Possible therapeutic applications. Eur. J. Biochem. 47: 179–185.
Sulkowski, W. W., D. Pentak, K. Nowak, and A. Sulkowska (2005) The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 744–747: 737–747.
Lian, T. and R. J. Y. Ho (2001) Trends and developments in liposome drug delivery system. J. Pharm. Sci. 90: 667–680.
Lapinski, M. M. A. Castro-Forero, A. J. Greiner, R. Y. Ofoli, and G. J. Blanchard (2007) Comparison of liposomes formed by sonciation and extrusion: Rotational and translational diffusion of an embedded chromophore. Langmuir 23: 11677–11683.
Gier, J. D., J. G. Mandersloot, and L. L. M. V. Deenen (1969) The role of cholesterol in lipid membranes. Biochim. Biophys. Acta. 173: 143–145.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bang, S.H., Sekhon, S.S., Kim, YH. et al. Preparation of liposomes containing lysosomal enzymes for therapeutic use. Biotechnol Bioproc E 19, 766–770 (2014). https://doi.org/10.1007/s12257-014-0327-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0327-7