Abstract
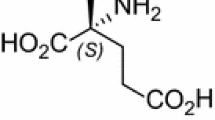
The enzymatic desymmetrization of 3-(4-fluorophenyl)glutaric anhydride (3-FGA) was investigated through lipase-catalyzed enantioselective alcoholysis in organic solvents. An immobilized Lipase B from Candida Antarctica (Novozym 435) was found to be an efficient biocatalyst for the enantioselective alcoholysis of 3-FGA. Methyl tert-butyl ether (MTBE) and methanol were chosen as the suitable reaction medium and acyl acceptor, respectively. The optimum reaction temperature, molar ratio of methanol to 3-FGA and 3-FGA concentration were 25°C, 2:1 and 100 mM, respectively. Under these conditions, complete conversion was achieved and methyl (S)-3-(4-fluorophenyl)glutarate ((S)-MFG) was obtained in a moderate ee value of 80%. Furthermore, the reaction was performed on a gram scale and the ee value of (S)-MFG was enriched to 96% after treatment with a toluene/hexane (2/1, v/v) mixture.
Similar content being viewed by others
Refrerences
García-Urdiales, E., I. Alfonso, and V. Gotor (2011) Update 1 of: Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev. 111: 110–180.
Palomo, J. M. and Z. Cabrera (2012) Enzymatic desymmetrization of prochiral molecules. Curr. Org. Synth. 9: 791–805.
López-García, M., I. Alfonso, and V. Gotor (2003) Desymmetrization of dimethyl 3-substituted glutarates through enzymatic ammonolysis and aminolysis reactions. Tetrahedron: Asymm. 14: 603–609.
Candy, M., G. Audran, H. Bienaymé, C. Bressy, and J.-M. Pons (2009) Enantioselective enzymatic desymmetrization of highly functionalized meso tetrahydropyranyl diols. Org. Lett. 11: 4950–4953.
Ríos-Lombardía, N., V. Gotor-Fernández, and V. Gotor (2011) Complementary lipase-mediated desymmetrization processes of 3-aryl-1,5-disubstituted fragments. Enantiopure synthetic valuable carboxylic acid derivatives. J. Org. Chem. 76: 811–819.
Sapu, C. M., J.-E. Bäckvall, and J. Deska (2011) Enantioselective enzymatic desymmetrization of prochiral allenic diols. Angew. Chem. Int. Ed. 50: 9731–9734.
Lu, X., L. Li, W. Yang, K. Jiang, K. F. Yang, Z. J. Zheng, and L. W. Xu (2013) Catalytic synthesis of functional silicon-stereogenic silanes through Candida antarctica lipase B catalyzed remote desymmetrization of silicon-centered diols. Eur. J. Org. Chem. 2013: 5814–5819.
Wang, B., J. Liu, X. L. Tang, C. Cheng, J. L. Gu, L. Y. Dai, and H. W. Yu (2010) Enzymatic synthesis of (S)-glutaric acid monoesters aided by molecular docking. Tetrahedron Lett. 51: 309–312.
Zutter, U., H. Iding, P. Spurr, and B. Wirz (2008) New, efficient synthesis of oseltamivir phosphate (Tamiflu) via enzymatic desymmetrization of a meso-1,3-cyclohexanedicarboxylic acid diester. J. Org. Chem. 73: 4895–4902.
Chênevert, R., F. Jacques, P. Giguèr, and M. Dasser (2008) Enzymatic desymmetrization of pyrrolidine and pyrroline derivatives. Tetrahedron: Asymm. 19: 1333–1338.
Yu, M. S., I. Lantos, Z.-Q. Peng, J. Yu, and T. Cacchio (2000) Asymmetric synthesis of (-)-paroxetine using PLE hydrolysis. Tetrahedron Lett. 41: 5647–5651.
Busto, E., V. Gotor-Fernández, J. Montejo-Bernardo, S. García-Granda, and V. Gotor (2007) First desymmetrization of 1,3-propanediamine derivatives in organic solvent. Development of a new route for the preparation of optically active amines. Org. Lett. 9: 4203–4206.
Ríos-Lombardía, N., E. Busto, E. García-Urdiales, V. Gotor-Fernández, and V. Gotor (2009) Enzymatic desymmetrization of prochiral 2-substituted-1,3-diamines: Preparation of valuable nitrogenated compounds. J. Org. Chem. 74: 2571–2574.
Wang, M. X., C. S. Liu, J. S. Li, and O. Meth-Cohn (2000) Microbial desymmetrization of 3-arylglutaronitriles, an unusual enhancement of enantioselectivity in the presence of additives. Tetrahedron Lett. 41: 8549–8552.
Wang, M. X., C. S. Liu, and J. S. Li (2001) Enzymatic desymmetrization of 3-alkyl-and 3-arylglutaronitriles, a simple and convenient approach to optically active 4-amino-3-phenylbutanoic acids. Tetrahedron: Asymm. 12: 3367–3373.
Fryszkowska, A., M. Komar, D. Koszelewski, and R. Ostaszewski (2005) Enzymatic desymmetrization of 3-arylglutaric acid anhydrides. Tetrahedron: Asymm. 16: 2475–2485.
Fryszkowska, A., J. Frelek, and R. Ostaszewski (2005) One-pot enzymatic desymmetrization and Ugi MCR. Tetrahedron 61: 6064–6072.
Fryszkowska, A., M. Komar, D. Koszelewski, and R. Ostaszewski (2006) Studies on enzymatic synthesis of chiral non-racemic 3-arylglutaric acid monoesters. Tetrahedron: Asymm. 17: 961–966.
Chênevert, R. and Y. S. Rose (2000) Enzymatic desymmetrization of a meso polyol corresponding to the C(19)-C(27) segment of rifamycin S. J. Org. Chem. 65: 1707–1709.
Liu, L. T., P.-C. Hong, H.-L. Huang, S.-F. Chen, C.-L. J. Wang, and Y.-S. Wen (2001) Asymmetric syntheses of trans-3,4-disubstituted 2-piperidinones and piperidines. Tetrahedron: Asymm. 12: 419–426.
Huang, X. J., S. Broadbent, C. Dvorak, and S. H. Zhao (2010) Pilot-Plant preparation of 3,4-dihydropyridin-2-one derivatives, the core structures of P2X7 receptor antagonists. Org. Proc. Res. Dev. 14: 612–616.
Liu, W. M., Y. Hu, L. Jiang, B. Zou, and H. Huang (2012) Synthesis of methyl (R)-3-(4-fluorophenyl)glutarate via enzymatic desymmetrization of a prochiral diester. Proc. Biochem. 47: 1037–1041.
Cabrera, Z., G. Fernandez-Lorente, J. M. Palomo, J. M. Guisan, and R. Fernandez-Lafuente (2008) Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase Ultra®: Effect of the immobilization protocol on its catalytic properties. Enz. Microb. Technol. 43: 531–536.
Chênevert, R. and M. Desjardins (1994) Chemoenzymatic enantioselective synthesis of baclofen. Can. J. Chem. 72: 2312–2317.
Homann, M. J., R. Vail, B. Morgan, V. Sabesan, C. Levy, D. R. Dodds, and A. Zaks (2001) Enzymatic hydrolysis of a prochiral 3-substituted glutarate Ester, an intermediate in the synthesis of an NK1/NK2 dual antagonist. Adv. Synth. Catal. 343: 744–749.
Huang, X. J., J. Zhu, and S. Broadbent (2010) The first asymmetric synthesis of a 4-aryl-substituted 5-carboxy-3,4-dihydropyridin-2-one derivative. Tetrahedron Lett. 51: 1554–1557.
Park, S. E., E. H. Nam, H. B. Jang, J. S. Oh, S. Some, Y. S. Lee, and C. E. Song (2010) Enantioselective alcoholysis of meso-glutaric anhydrides catalyzed by Cinchona-based sulfonamide catalysts. Adv. Synth. Catal. 352: 2211–2217.
Fitzpatrick, P. A. and A. M. Klibanov (1991) How can the solvent affect enzyme enantioselectivity? J. Am. Chem. Soc. 113: 3166–3171.
Valivety, R. H., G. A. Johnston, C. J. Suckling, and P. J. Halling (1991) Solvent effects on biocatalysis in organic Systems: Equilibrium position and rates of lipase catalyzed esterification. Biotechnol. Bioeng. 38: 1137–1143.
Ducret, A., M. Trani, and R. Lortie (1998) Lipase-catalyzed enantioselective esterification of ibuprofen in organic solvents under controlled water activity. Enz. Microb. Technol. 22: 212–216.
Ozegowski, R., A. Kunath, and H. Schick (1995) The different behaviour of syn-and anti-2,3-dimethylbutanedioic anhydride in the lipase-catalyzed enantioselective alcoholysis. Tetrahedron: Asymm. 6: 1191–1194.
Ozegowski, R., A. Kunath, and H. Schick (1993) Lipase-catalyzed asymmetric alcoholysis of 3-substituted pentanedioic anhydrides. Liebigs Ann. Chem. 1993: 805–808.
Zhao, D. T., E. N. Xun, J. X. Wang, R. Wang, X. F. Wei, L. Wang, and Z. Wang (2011) Enantioselective esterification of ibuprofen by a novel thermophilic biocatalyst: APE1547. Biotechnol. Bioproc. Eng. 16: 638–644.
Manzano, R., J. M. Andrés, M.-D. Muruzábal, and R. Pedrosa (2010) Synthesis of both enantiomers of hemiesters by enantioselective methanolysis of meso cyclic anhydrides catalyzed by a-amino acid-derived chiral thioureas. J. Org. Chem. 75: 5417–5420.
Huang, J., F. Xiong, and F. E. Chen (2008) Total synthesis of (+)-biotin via a quinine-mediated asymmetric alcoholysis of mesocyclic anhydride strategy. Tetrahedron: Asymm. 19: 1436–1443.
Naik, S., A. Basu, R. Saikia, B. Madan, P. Paul, R. Chaterjee, J. Brask, and A. Svendsen (2010) Lipases for use in industrial biocatalysis: Specificity of selected structural groups of lipases. J. Mol. Catal. B: Enzym. 65: 18–23.
Wolff, A., L. Zhu, Y. W. Wong, A. J. J. Straathof, J. A. Jongejan, and J. J. Heijnen (1999) Understanding the influence of temperature change and cosolvent addition on conversion rate of enzymatic suspension reactions based on regime analysis. Biotechnol. Bioeng. 62: 125–134.
Jin, Z., S. L. Liang, X. Q. Zhang, S. Y. Han, C. Q. Ren, Y. Lin, and S. P. Zheng (2013) Synthesis of fructose laurate esters catalyzed by a CALB-displaying Pichia pastoris whole-cell biocatalyst in a non-aqueous system. Biotechnol. Bioproc. Eng. 18: 365–374.
Dong, H. P., Y. J. Wang, and Y. G. Zheng (2010) Enantioselective hydrolysis of diethyl 3-hydroxyglutarate to ethyl (S)-3-hydroxyglutarate by immobilized Candida antarctica lipase B. J. Mol. Catal. B: Enzym. 66: 90–94.
Li, X., T. Liu, L. Xu, X. H. Gui, F. Su, and Y. J. Yan (2012) Resolution of racemic ketoprofen in organic solvents by lipase from Burkholderia cepacia G63. Biotechnol. Bioproc. Eng. 17: 1147–1155.
Foresti, M. L., M. Galle, M. L. Ferreira, and L. E. Briand (2009) Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 84: 1461–1473.
Ramamurthi, S. and A. R. McCurdy (1994) Lipase-catalyzed esterification of oleic acid and methanol in hexane-A kinetic study. J. Am. Oil Chem. Soc. 71: 927–930.
Zhang, H. Y., X. Wang, and C. B. Ching (2007) R-stereopreference analysis of lipase Novozym 435 in kinetic resolution of flurbiprofen. Chiral. 19: 245–249.
Wu, J.-Y. and S.-W. Liu (2000) Influence of alcohol concentration on lipase-catalyzed enantioselective esterification of racemic naproxen in isooctane: Under controlled water activity. Enz. Microb. Technol. 26: 124–130.
Xue, Y. P., T. Jiang, X. Liu, and Y. G. Zheng (2013) Efficient production of S-(+)-2-chlorophenylglycine by immobilized penicillin G acylase in a recirculating packed bed reactor. Biochem. Eng. J. 74: 88–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Hu, Y., Zhang, Y. et al. Enzymatic desymmetrization of 3-(4-fluorophenyl)glutaric anhydride through enantioselective alcoholysis in organic solvents. Biotechnol Bioproc E 19, 449–455 (2014). https://doi.org/10.1007/s12257-014-0110-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-014-0110-9