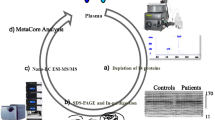
Abstract
The aim of this study was to screen for proteins that are susceptible to glycation under hyperglycemic conditions in patients with type 2 diabetic nephropathy. Serum proteins were analyzed by a proteomic approach using two-dimensional electrophoresis (2-DE) and ESI-Q-TOF MS/MS. Gels were stained with Pro-Q Emerald 488 to analyze the serum glycoproteome, followed by silver nitrate to examine the total serum proteome. Patient sera were divided into four groups according to their microalbuminuria index: type 2 diabetics with normoalbuminuria, microalbuminuria, and overt nephropathy, and healthy subjects. When the HbA1c levels of the diabetic groups were examined, groups with higher HbA1c exhibited higher fructosamine levels, suggesting that the loss of glycemic control affected the glycation of serum proteins. The proteins that became glycated under poor glycemic control were PEDF, apolipoprotein J precursor, hemopexin, immunoglobulin mu heavy chain, and immunoglobulin kappa chain. As albuminuria increased, a marker of kidney damage, the levels of glycated prekallikrein and complement factor C4B3 also increased. The glycated proteins identified in this study may provide the foundation for the development of novel markers of diabetes, hyperglycemia, and diabetic complications.
Similar content being viewed by others
References
Korc, M. (2003) Diabetes mellitus in the era of proteomics. Mol. Cell Proteomics 2: 399–404.
Abou-Seif, M. A. and A. A. Youssef (2004) Evaluation of some biochemical changes in diabetic patients. Clin. Chim. Acta 346: 161–170.
Brownlee, M. (1995) The pathological implications of protein glycation. Clin. Invest. Med. 18: 275–281.
Brownlee, M. (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820.
Ritz, E., I. Rychlik, F. Locatelli, and S. Halimi (1999) End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am. J. Kidney Dis. 34: 795–808.
Rao, P. V., X. Lu, M. Standley, P. Pattee, G. Neelima, G. Girisesh, K. V. Dakshinamurthy, C. T. Roberts, and S. R. Nagalla (2007) Proteomic identification of urinary biomarkers of diabetic nephropathy. Diab. Care 30: 629–637.
Steinke, J. M., A. R. Sinaiko, M. S. Kramer, S. Suissa, B. M. Chavers, M. Mauer and International Diabetic Nephropathy Study Group (2005) The early natural history of nephropathy in Type 1 diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171.
Matheson, A., M. D. Willcox, J. Flanagan, and B. J. Walsh (2010) Urinary biomarkers involved in type 2 diabetes: A review. Diab. Metab. Res. Rev. 26: 150–171.
Ahmed, N. (2005) Advanced glycation endproducts—role in pathology of diabetic complications. Diab. Res. Clin. Pract. 67: 3–21.
Raj, D. S., D. Choudhury, T. C. Welbourne, and M. Levi (2000) Advanced glycation end products: A Nephrologist’s perspective. Am. J. Kidney Dis. 35: 365–380.
Ulrich, P. and A. Cerami (2001) Protein glycation, diabetes, and aging. Recent Prog. Horm. Res. 56: 1–21.
Jaleel, A., P. Halvatsiotis, B. Williamson, P. Juhasz, S. Martin, and K. S. Nair (2005) Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diab. Care 28: 645–652.
Schalkwijk, C. G., N. Chaturvedi, H. Twaafhoven, V. W. van Hinsbergh, and C. D. Stehouwer (2002) Amadori-albumin correlates with microvascular complications and precedes nephropathy in type 1 diabetic patients. Eur. J. Clin. Invest. 32: 500–506.
Chase, H. P., W. E. Jackson, S. L. Hoops, R. S. Cockerham, P. G. Archer, and D. O’Brien (1989) Glucose control and the renal and retinal complications of insulin-dependent diabetes. JAMA 261: 1155–1160.
Sullivan, K. A. and E. L. Feldman (2005) New developments in diabetic neuropathy. Curr. Opin. Neurol. 18: 586–590.
Allgrove, J. and B. L. Cockrill (1988) Fructosamine or glycated haemoglobin as a measure of diabetic control? Arch. Dis. Child. 63: 418–422.
Zhang, Q., N. Tang, A. A. Schepmoes, L. S. Phillips, R. D. Smith, and T. O. Metz (2008) Proteomic profiling of nonenzymatically glycated proteins in human plasma and erythrocyte membranes. J. Proteome Res. 7: 2025–2032.
Ryu, J. K., H. S. Kim, and D. H. Nam (2012) Current status and perspectives of biopharmaceutical drugs. Biotechnol. Bioproc. Eng. 17: 900–911.
Cheng, J. S., X. M. Lv, and Y. J. Yuan (2012) Investigation of proteomic responses of streptomyces lydicus to pitching ratios for improving streptolydigin production. Biotechnol. Bioproc. Eng. 17: 997–1007.
Bradford, M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Baker, J. R., D. V. Zyzak, S. R. Thorpe, and J. W. Baynes (1994) Chemistry of the fructosamine assay: D-glucosone is the product of oxidation of Amadori compounds. Clin. Chem. 40: 1950–1955.
Johnson, R. N., P. A. Metcalf, and J. R. Baker (1983) Fructosamine: A new approach to the estimation of serum glycosylprotein; An index of diabetic control. Clin. Chim. Acta 127: 87–95.
Kim, H. J., E. H. Cho, J. H. Yoo, P. K. Kim, J. S. Shin, M. R. Kim, and C. W. Kim (2007) Proteome analysis of serum from type 2 diabetics with nephropathy. J. Proteome. Res. 6: 735–743.
Cho, E. H., M. R. Kim, H. J. Kim, D. Y. Lee, P. K. Kim, K. M. Choi, O. H. Ryu, and C. W. Kim (2007) The discovery of biomarkers for type 2 diabetic nephropathy by serum proteome analysis. Proteomics-Clin. Appl. 1: 352–361.
Shurraw, S., B. Hemmelgarn, M. Lin, S. R. Majumdar, S. Klarenbach, B. Manns, A. Bello, M. James, T. C. Turin, and M. Tonelli (2011) Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease. Arch. Intern. Med. 171: 1920–1927.
Lapolla, A., L. Molin, and P. Traldi (2013) Protein glycation in diabetes as determined by mass spectrometry. Int. J. Endocrinol. 2013: 11–21.
Tombran-Tink, J. and L. V. Johnson (1989) Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest. Ophthalmol. Vis. Sci. 30: 1700–1707.
Jenkins, A., S. X. Zhang, A. Gosmanova, C. Aston, A. Dashti, M. Z. Baker, T. Lyons, and J. X. Ma (2008) Increased serum pigment epithelium derived factor levels in tye 2 diabetes patients. Diab. Res. Clin. Pr. 82: 5–7.
Chen, H., Z. Zheng, R. Li, J. Lu, Y. Bao, X. Ying, R. Zeng, and W. Jia (2010) Urinary pigment epithelium-derived factor as a marker of diabetic nephropathy. Am. J. Nephrol. 32: 47–56.
Müller-Eberhard, U. (1988) Hemopexin. Methods Enzymol. 163: 536–565.
Zhang, Q., M. E. Monroe, A. A. Schepmoes, T. R. W. Clauss, M. A. Gritsenko, D. Meng, V. A. Petyuk, R. D. Smith, and T. O. Metz (2011) Comprehensive identification of glycated peptides and their glycation motifs in plasma and erythrocytes of control and diabetic subjects. J. Proteome Res. 10: 3076–3088.
Trougakos, I. P., M. Poulakou, M. Stathatos, A. Chalikia, A. Melidonis, and E. S. Gonos (2002) Serum levels of the senescence biomarker clusterin/apolipoprotein J increase significantly in diabetes type II and during development of coronary heart disease or at myocardial infarction. Exp. Gerontol. 37: 1175–1187.
Jaffa, A. A., R. Durazo-Arvizu, D. Zheng, D. T. Lackland, S. Srikanth, W. T. Garvey, and A. H. Schmaier (2003) Plasma prekallikrein: Plasma prekallikrein: A risk marker for hypertension and nephropathy in type 1 diabetes. Diabetes 52: 1215–1221.
Herwald, H., T. Renné, J. C. M. Meijers, D. W. Chung, J. D. Page, R. W. Colman, and W. Müller-Esterl (1996) Mapping of the discontinuous kininogen binding site of prekallikrein. A distal binding segment is located in the heavy chain domain A4. J. Bio. Chem. 271: 13061–13067.
Kedzierska, K., K. Ciechanowski, E. Gołembiewska, K. Safranow, A. Ciechanowicz, L. Domański, M. Myślak, and J. Róźański (2005) Plasma prekallikrein as a risk factor for diabetic retinopathy. Arch. Med. Res. 36: 539–543.
Mijovic, C., J. Fletcher, A. R. Bradwell, T. Harvey, and A. H. Barnett (1985) Relation of gene expression (allotypes) of the fourth component of complement to insulin dependent diabetes and its microangiopathic complications. BMJ 291: 9–10.
Yamagishi, S., T. Matsui, H. Adachi, and M. Takeuchi (2010) Positive association of circulating levels of advanced glycation end products (AGEs) with pigment epithelium-derived factor (PEDF) in a general population. Pharmacol. Res. 61: 103–107.
Yamagishi, S., T. Matsui, K. Nakamura, M. Takeuchi, and T. Imaizumi (2006) Pigment epithelium-derived factor (PEDF) prevents diabetes-or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc. Res. 72: 86–90.
Muranjan, M., V. Nussenzweig, and S. Tomlinson (1998) Characterization of the human serum Trypanosome toxin, Haptoglobin-related Protein. J. Bio. Chem. 273: 3884–3887.
Epelbaum, R., C. Shalitin, R. Segal, C. Valansi, I. Arselan, D. Faraggi, M. M. Leviov, M. B. Shahar, and N. Haim (1998) Haptoglobin-related protein as a serum marker in malignant lymphoma. Pathol. Oncol. Res. 4: 271–276.
Asleh, R. and A. P. Levy (2005) In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vas Heal. Risk Manag. 1: 19–28.
Samir, M. A., A. S. Suleiman, M. A. S. Qasem, and H. Hisham (2008) Association of haptoglobin phenotypes with markers of diabetic nephropathy in Type 2 diabetes mellitus. J. Diab. Compl. 22: 384–388.
Phadke, M., F. R. Billimoria, and V. Ninjoor (1998) Non enzymatic glycosylation of alpha-1-proteinase inhibitor of human plasma. J. Postgrad. Med. 44: 29–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, MR., Yu, SA., Kim, MY. et al. Analysis of glycated serum proteins in type 2 diabetes patients with nephropathy. Biotechnol Bioproc E 19, 83–92 (2014). https://doi.org/10.1007/s12257-013-0464-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-013-0464-4