Abstract
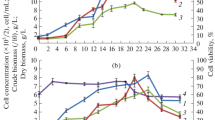
Cyclocarya paliurus is a unique plant growing in central China with hypoglycaemic and hypolipaemia effects. To make better use of this functional food resource, cell suspension cultures and triterpenic acid accumulation were studied. Stable and uniform cell suspension cultures were established in liquid basal Murashige and Skoog medium supplemented with 2,4-dichlorophenoxy acetic acid (0.5 mg/L), naphthalene acetic acid (0.3 mg/L) and cytokinin (1.0 mg/L). According to the growth curve and triterpenic acid accumulation curve, the 8 ∼ 10th day postinoculation was the optimum time for subculture, and the 14th day was the optimum time for harvest. Murashige and Skoog medium and woody plant medium were suitable for both cell growth and triterpenic acid accumulation. 3% sucrose (w/v), 60 mM total nitrogen (NO3 −/NH4 + = 2/1), 1.25 mM KH2PO4, 2 mM CaCl2, and 2 mM MgSO4 were all found to be fit for cell growth and triterpenic acid accumulation in a cell suspension culture of Cyclocarya paliurus. Total triterpenic acid, ursolic acid and oleanolic acid content in suspended cultured cells were all significantly higher than that of leaves and calluses (P ⩽ 0.01), with levels up to 6.24, 2.28, and 0.94% (of dry weight), respectively. The betulinic acid content of suspended cultured cells also reached 0.82%, which was significantly higher than that of calluses. These results suggest that suspended cultured cells of Cyclocarya paliurus were rich in triterpenic acids and could be used for the production of total triterpenic acid, ursolic acid, oleanolic acid and betulinic acid.
Similar content being viewed by others
References
Xie, M. Y. and J. H. Xie (2008) Review about the research on Cyclocarya paliurus (Batal.) Iljinskaja. J. Food Sci. Biotechnol. 27: 113–121.
Yin, Z. P., X. C. Shangguan, D. M. Li, S. F. Wu, J. G. Chen, and Y. H. Zhang (2010) A study on ultrasonic-assisted extraction of total triterpenoids from Cyclocarya paliurus leaves. Acta Agriculturae Universitati 32: 373–377.
Fang, S. Z., W. X. Yang, X. L. Chu, X. L. Shang, C. Q. She, and X. X. Fu (2011) Provenance and temporal variations in selected flavonoids in leaves of Cyclocarya paliurus. Food Chem. 124: 1382–1386.
Xie, J. H., M. Y. Xie, S. P. Nie, M. Y. Shen, Y. X. Wang, and C. Li (2010) Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 119: 1626–1632.
Fang, S. Z., X. L. Chu, X. L. Shang, W. X. Yang, X. X. Fu, and C. Q. She (2011) Seasonal variation of microelement contents in leaves of Cyclocarya paliurus among the provenances. J. Forest Res. 22: 225–231.
Xie, M. Y., L. Li, S. P. Nie, X. R. Wang, and Frank S. C. Lee (2006) Determination of speciation of elements related to blood sugar in bioactive extracts from Cyclocarya paliurus leaves by FIA-ICP-MS. Eur. Food Res. Technol. 223: 202–209.
Li, S., J. Li, X. L. Guan, J. Li, M. T. Deng, J. G. Huang, Z. Z. Chen, and R. Y. Yang (2011) Hypoglycemic effects and constituents of the barks of Cyclocarya paliurus and their inhibiting activities to glucosidase and glycogen phosphorylase. Fitoterapia 82: 1081–1085.
Zhang, J., Q. Shen, J. C. Lu, J. Y. Li, W. Y. Liu, J. J. Yang, J. Li, and K. Xiao (2010) Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 119: 1491–1496.
Kurihara, H., H. Fukami, A. Kusumoto, Y. Toyoda, H. Shibata, Y. Matsui, S. Asami, and T. Tanaka (2003) Hypoglycemic action of Cyclocarya paliurus (Batal.) Iljinskaja in normal and diabetic mice. Biosci. Biotechnol. Biochem. 67: 877–880.
Kurihara, H., S. Asami, H. Shibata, H. Fukami, and T. Tanaka (2003) Hypolipemic effect of Cyclocarya paliurus (Batal.) Iljinskaja in lipid-loaded mice. Biol. Pharm. Bull. 26: 383–385.
Xie, J. H., M.Y. Shen, M. Y. Xie, S. P. Nie, Y. Chen, C. Li, D. F. Huang, and Y. X. Wang (2012) Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohyd. Polym. 89: 177–184.
Shu, R. G., C. R. Xu, L. N. Li, and Z. L. Yu (1995) Cyclocariosides II and III: Two secodammarane triterpenoid saponins from Cyclocarya paliurus. Planta Med. 61: 551–553.
Li, S. A., B. S. Cui, Q. Liu, L. Tang, Y. C. Yang, X. J. Jin, and Z. F. Shen (2012) New triterpenoids from the leaves of Cyclocarya paliurus. Planta Med. 78: 290–296.
Messner, B., I. Zeller, C. Ploner, S. Frotschnig, T. Ringer, A. Steinacher-Nigisch, A. Ritsch, G. Laufer, C. Huck, and D. Bernhard (2011) Ursolicacid causes DNA-damage, P53-mediated, mitochondria- and caspase-dependent human endothelial cell apoptosis, and accelerates atherosclerotic plaque formation in vivo. Atherosclerosis 219: 402–408.
Buus, N. H., N. C. Hansson, R. Rodriguez-Rodriguez, E. Stankevicius, M. R. Anderson, and U. Simonsen (2011) Antiatherogenic effects of oleanolicacid in apolipoprotein E knockout mice. Eur. J. Pharmacol. 670: 519–526.
Ghosh, J., J. Das, P. Manna, and P. C. Sil (2011) The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials 32: 4857–4866.
Fang, S. Z., J. Y. Wang, Z. Y. Wei, and Z. X. Zhu (2006) Methods to break seed dormancy in Cyclocarya paliurus (Batal) Iljinskaja. Scientia Horticult. 110: 305–309.
Georgiev, M. I., J. Weber, and A. Maciuk (2009) Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 83: 809–823.
Ramachandra Rao, S. and G. A. Ravishankar (2002) Plant cell cultures: Chemical factories of secondary metabolites. Biotech. Adv. 20: 101–153.
Bonfill, M., S. Mangas, E. Moyano, R. M. Cusido, and J. P. Palazon (2011) Production of centellosides and phytosterols in cell suspension cultures of Centella asiatica. Plant Cell Tiss. Organ. Cult. 104: 61–67.
Nagella, P. and H. N. Murthy (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide-A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tiss. Organ. Cult. 104: 119–124.
Srivastava, P., N. Kasoju, U. Bora, and R. Chaturvedi (2010) Accumulation of betulinic, oleanolic, and ursolic acids in in vitro cell cultures of Lantana camara L. and their significant cytotoxic effects on heLa cell lines. Biotechnol. Bioproc. Eng. 15: 1038–1046.
Srivastava, P., V. Sisodia, and R. Chaturvedi (2011) Effect of cul- ture conditions on synthesis of triterpenoids in suspension cultures of Lantana camara L. Bioproc. Biosyst. Eng. 34: 75–80.
Murashige, T. and F. Skoog (1962) A revised medium for rapid growth and bioassays with tobacoo tissue cultures. Physiol. Plant 15: 473–497.
Kolewe, M. E., M. A. Henson, and S. C. Roberts (2010) Characterization of aggregate size in Taxus suspension cell culture. Plant Cell Rep. 29: 485–494.
Forni, C., A. Frattarelli, and C. Damiano (1999) Different size, shape and growth behavior of cells in suspension cultures of strawberry (Fragaria × ananassa Duch.). Plant Biosyst. 133: 205–212.
Edahiro, J. and M. Seki (2006) Phenylpropanoid metabolite supports cell aggregate formation in strawberry cell suspension culture. J. Biosci. Bioeng. 102: 8–13.
Boonsnongcheep, P., S. Korsangruang, N. Soonthornchareonnon, Y. Chintapakorn, P. Saralamp, and S. Prathanturarug (2010) Growth and isoflavonoid accumulation of Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tiss. Organ Cult. 101: 119–126.
Yang, Y., F. He, and L. J. Yu (2008) Dynamics analyses of nutrients consumption and flavonoids accumulation in cell suspension culture of Glycyrrhiza inflata. Biol. Plantarum 52: 732–734.
Nagella, P. and H. N. Murthy (2010) Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresource Technol. 101: 6735–6739.
Simões, C., L. Silva Cordeiro, T. C. Castro, C. H. Callado, N. Albarello, and E. Mansur (2011) Establishment of anthocyaninproducing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tiss. Organ Cult. 106: 537–545.
Lian, M. L., D. Chakrabarty, and K. Y. Paek (2002) Effect of plant growth regulators and medium composition on cell growth and saponin production during cell suspension culture of mountain ginseng (Panax ginseng C.A. Mayer). J. Plant Biol. 45: 201–206.
Naik, P. M., S. H. Manohar, and H. N. Murthy (2011) Effects of macro elements and nitrogen source on biomass accumulatin and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol. Plant 33: 1553–1557.
Prakash, G. and A. K. Srivastava (2005) Statistical media optimization for cell growth and azadirachtin production in Azadirachta indica (A. Juss) suspension cultures. Proc. Biochem. 40: 3795–3800.
Pan, X. W., H. H. Xu, X. Liu, X. Gao, and Y. T. Lu (2004) Improvement of growth and camptothecin yield by altering nitrogen source supply in cell suspension cultures of Camptotheca acuminata. Biotechnol. Lett. 26: 1745–1748.
Cordts, J. M., R. Scorza, and R. L. Bell (1987) Effects of carbohydrates and nitrogen on the development of anthocyanins of a red leaf peach (Prunus persica (L.) Batsch) in vitro. Plant Cell Tiss. Organ Cult. 9: 103–110.
Yamada, J., K. Fujita, K. Eto, and K. Sakai (2003) Cell growth and nutrient uptake by cell suspensions of Cupressus lusitanica. J. Wood Sci. 49: 5–10.
Shin, K. S., D. Chakrabarty, J. Y. Ko, S. S. Han, and Y. K. Paek (2003) Sucrose utilization and mineral nutrient uptake during hairy root growth of red beet (Beta vulgaris L.) in liquid culture. Plant Growth Regul. 39: 187–193.
Yu, K. W., W. Y. Gao, E. J. Hahn, and K. Y. Paek (2001) Effects of macro elements and nitrogen source on adventitious root growth and ginsenoside production in ginseng (Panax ginseng C. A. Meyer). J. Plant Biol. 44: 179–184.
Weathers, P. J., M. J. Towler, and J. F. Xu (2010) Bench to batch: Advances in plant cell culture for producing useful products. Appl. Microbiol. Biotechnol. 85: 1339–1351.
Huang, T. K. and K. A. McDonald (2009) Bioreactor engineering for recombinant protein production in plant cell suspension cultures. Biochem. Eng. J. 45: 168–184.
Smetanska, I. (2008) Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng. Biotechnol. 111: 187–228.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Z., Shangguan, X., Chen, J. et al. Growth and triterpenic acid accumulation of Cyclocarya paliurus cell suspension cultures. Biotechnol Bioproc E 18, 606–614 (2013). https://doi.org/10.1007/s12257-012-0751-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-012-0751-5