Abstract
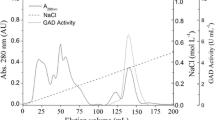
Gene encoding for a putative glutamate decarboxylase (GAD: EC 4.1.1.15) from the hyperthermophilic archaeon Pyrococcus furiosus was cloned and the biochemical characteristics of the resulting recombinant protein were examined. The gene (PF1159) from P. furiosus showed some identity with other group II decarboxylases from an archaea and bacteria. The GAD from P. furiosus (PfGAD) was expressed in Escherichia coli, and the recombinant protein has a molecular mass of 41 kDa, determined by SDS-PAGE. The optimum temperature and pH for GAD activity were 75°C and 6.0, respectively. The half-life of heat inactivation was approximately 60 min at 90°C. The GAD activity was found to be dependent on various salts, such as CaCl2, NaCl, KCl, and NaBr, with an optimum concentration of 400 mM, but not (NH4)2SO4. PfGAD demonstrated activity against various substrates, such as l-glutamate, l-aspartate, and l-tyrosine. The results of the kinetics experiment indicated that l-aspartate was a better substrate of PfGAD than l-glutamate and Ltyrosine.
Similar content being viewed by others
References
Robert, E. and S. Frankel (1950) γ-Aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 187: 55–63.
Bazemore, A. W., K. A. C. Elliott, and E. Florey (1957) Isolation of factor I. J. Neurochem. 1: 334–339.
Stanton, H. C. (1963) Mode of action of gamma aminobutyric acid on the cardiovascular system. Arch. Int. Pharmacodyn. 143: 195–200.
Omori, M., T. Yano, J. Okamoto, T. Tsushida, T. Murai, and H. Higuchi (1987) Effect of anaerobically treated tea (gabaron tea) on blood pressure of spontaneously hypertensive rats. Nippon Nogeikagaku Kaishi. 61: 1449–1451.
Adeghate, E. and A. S. Ponery (2002) GABA in the endocrine pancreas: Cellular localization and function in normal and diabetic rats. Tissue Cell. 34: 1–6.
Hagiwara, H., T. Seki, and T. Ariga (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 68: 444–447.
Ueno, H. (2000) Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. 10: 67–79.
Kim, H. W., Y. Kashima, K. Ishikawa, and N. Yamano (2009) Purification and characterization of the first archaeal glutamate decarboxylase from Pyrococcus horikoshii. Biosci. Biotechnol. Biochem. 73: 224–227.
Gut, H., E. Pennacchietti, R. A. John, F. Bossa, G. Capitani, D. De. Biase, and M. G. Grutter (2006) Escherichia coli acid resistance: pH-sensing, activation by chloride and autoinhibition in GadB. EMBO J. 25: 2643–2651.
Hiraga, K., Y. Ueno, and K. Oda (2008) Glutamate decarboxylase from Lactobacillus brevis: Activation by ammonium sulfate. Biosci. Biotechnol. Biochem. 72: 1299–1306.
Kobayashi, S., W. Tsuzuki, K. Yamamoto, T. Tsushida, T. Ishizuka, K. Hiwatari, K. Moruya, and K. Moriya (1999) Japan Patent 2,891,296.
Hayakawa, K., Y. Ueno, S. Azumagushi, and B. Kim (2006) Japan Patent 3,880,820.
Hong, S. J., I. Ullah, and G. S. Park (2012) Overexpression and Characterization of Recombinant Glutamate Decarboxylase from Thermococcus kodakaraensis KOD1. J. Kor. Soc. Appl. Biol. Chem. 55: 213–218.
Brown, S. H. and R. M. Kelly (1989) Cultivation techniques for hyperthermophilic archaebacteria: Continuous culture of Pyrococcus furiosus at temperatures near 100°C. Appl. Environ. Microbiol. 55: 2086–2088.
Polyakova, Y., Y. M. Koo, and K. H. Row (2006) Application of ionic liquids as mobile phase modifier in HPLC. Biotechnol. Bioproc. Eng. 11: 1–6.
Perrella, F. W. (1988) EZ-FIT: A practical curve-fitting micro-computer program for the analysis of enzyme kinetic data on IBM-PC compatible computers. Anal. Biochem. 174: 437–447.
Maeder D. L., R. B. Weiss, D. M. Dunn, J. L. Cherry, J. M. González, J. DiRuggiero, and F. T. Robb (1999) Divergence of the hyperthermophilic archaea Pyrococcus furiosus and P. horikoshii inferred from complete genomic sequences. Gen. 152: 1299–1305.
Hersh, B. M., F. T. Farooq, D. N. Barstad, D. L. Blankenhorn, and J. L. Slonczewski (1996) A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 178: 3978–3981.
Ueno, Y., K. Hayakawa, S. Takahashi, and K. Oda (1997) Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci. Biotechnol. Biochem. 61: 1168–1171.
Murzin, A. G. (1996) Structural classification of proteins: New superfamilies. Curr. Opin. Struct. Biol. 6: 386–394.
Burkhard, P., P. Dominici, C. Borri-Voltattorni, J. N. Jansonius, and V. N. Malashkevich (2001) Structural insight into Parkinson’s disease treatment from drug-inhibited DOPA decarboxylase. Nat. Struct. Biol. 8: 963–967.
Momany, C., R. Ghosh, and M. L. Hackert (1995) Structural motifs for pyridoxal-5′-phosphate binding in decarboxylases: An analysis based on the crystal structure of the Lactobacillus 30a ornithine decarboxylase. Protein Sci. 4: 849–854.
Kawalleck, P., H. Keller, K. Hahlbrock, D. Scheel, and I. E. Somssich (1993) A pathogen-responsive gene of parsley encodes tyrosine decarboxylase. J. Biol. Chem. 268: 2189–2194.
Hao, R. and J. C. Schmit (1991) Purification and characterization of glutamate decarboxylase from Neurospora crassa conidia. J. Biol. Chem. 266: 5135–5139.
Capitani, G., D. De. Biase, C. Aurizi, H. Gut, F. Bossa, and M. G. Grütter (2003) Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 22: 4027–4037.
Yap, K. L., T. Yuan, T. K. Mal, H. J. Vogel, and M. Ikura (2003) Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J. Mol. Biol. 328: 193–204.
Nishioka, M., K. Tanimoto, N. Higashi, H. Fukada, K. Ishikawa, and M. Taya (2008) Alteration of metal ions improves the activity and thermostability of aminoacylase from hyperthermophilic archaeon Pyrococcus horikoshii. Biotechnol. Lett. 30: 1639–1643.
Jeon, S. J. and K. Ishikawa (2005) Characterization of the Family I inorganic pyrophosphatase from Pyrococcus horikoshii OT3. Archaea. 1: 385–389.
Gut, H., E. Pennacchietti, R. A. John, F. Bossa, G. Capitani, D. De. Biase, and M. G. Grutter (2006) Escherichia coli acid resistance: pH-sensing, activation by chloride and autoinhibition in GadB. EMBO J. 25: 2643–2651.
Hasan, M. N., P. L. Hagedoorn, and W. R. Hagen (2002) Pyrococcus furiosus ferredoxin is a functional dimer. FEBS Lett. 531: 335–338.
Komatsuzaki, N., T. Nakamura, T. Kimura, and J. Shima (2008) Characterization of glutamate decarboxylase from a high gammaaminobutyric acid (GABA)-producer, Lactobacillus paracasei. Biosci. Biotechnol. Biochem. 72: 278–285.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, ES., Kim, HW., Kim, DE. et al. Gene expression and characterization of thermostable glutamate decarboxylase from Pyrococcus furiosus . Biotechnol Bioproc E 18, 375–381 (2013). https://doi.org/10.1007/s12257-012-0581-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-012-0581-5