Abstract
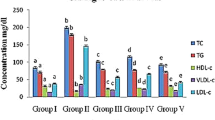
The hypoglycemic effect of the crude extracellular polysaccharides (EPS) produced from submerged mycelial culture of an edible mushroom Laetiporus sulphureus var. miniatus in streptozotocin (STZ)-induced diabetic rat was investigated. Hypoglycemic effect of EPS was evaluated in STZ-induced diabetic rats, and its possible mechanism was suggested by the results of western blot analysis and immunohistochemical staining. The results revealed that orally administrated EPS, when given 48 h after STZ treatment exhibited an excellent hypoglycemic effect, lowering the average plasma glucose level in EPS-fed rats to 43.5% of STZ-treated rats. The plasma levels of total cholesterol and triglyceride were significantly increased upon STZ treatment and they were markedly reduced by oral administration of EPS to near-normal levels. The results of immunohistochemical staining of the pancreatic tissues showed that EPS treatment considerably increased the insulin antigenesity of diabetic islet β-cells, suggesting the possibility of β-cell proliferation or regeneration by EPS therapy. Moreover, immunoblotting study revealed that protein levels of iNOS was increased and SOD2, catalase, GPx were significantly increased after EPS treatments, suggesting alleviated oxidative stress mediated by STZ. Orally administrated EPS exhibited considerable hypoglycemic effect in STZ-induced diabetic rats and that these EPS may be useful for the management of diabetes mellitus.
Similar content being viewed by others
References
Kawasaki, E., N. Abiru, and K. Eguchi (2004) Prevention of type 1 diabetes: from the view point of β-cell damage. Diabetes. Res. Clin. Pract. 66: S27–S32.
Khan, K. and M. Safdar (2003) Role of diet, nutrients, spices, and natural products in diabetes mellitus. Asia Pac. J. Clin. Nutr. 2: 1–12.
Gray, A. M. and P. R. Flatt (1997) Nature’s own pharmacy: the diabetes perspective. Proc. Nutr. Soc. 56: 507–517.
Lamba, S. S., K. Y. Buch, H. Lewis, and J. Lamba, (2000) Phytochemicals as potential hypoglycemic agents. Studies in Natural Products Chemistry 21: 457–496.
Grover, J. K., S. Yadav, and V. Vats (2002) Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 81: 81–100.
Li, W. L., H. C. Zheng, J. Bukuru, and N. De Kimpe (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 92: 1–21.
Kiho, T., A. Yamane, J. Hui, S. Usui, and S. Ukai (1996) Polysaccharide in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effects on glucose metabolism in mouse liver. Biol. Pharm. Bull. 19: 294–296.
Kiho, T., K. Ookubo, S. Usui, S. Ukai, and K. Hirano (1999) Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 22: 966–970.
Gray, A. M. and P. R. Flatt (1998) Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). J. Endocrinol. 157: 259–266.
Lo, H. C., S. T. Tu, K. C. Lin, and S. C. Lin (2004) The anti-hyperglycemic activity of the fruiting body of Cordyceps in diabetic rats induced by nicotinamide and streptozotocin. Life Sci. 74: 2897–2908.
Zhang, H. N. and Z. B. Lin (2004) Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta. Pharmacol. Sin. 25: 191–195.
Hu, S. H., J. C. Wang, J. L. Lien, E. T. Liaw, and M. Y. Lee (2006) Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl. Microbiol. Biotechnol. 70: 107–113.
Kim, D. H., B. K. Yang, S. C. Jeong, N. J. Hur, S. Das, J. W. Yun, J. W. Choi, Y. S. Lee, and C. H. Song (2001) A preliminary study on the hypoglycemic effect of the exo-polymers produced by five different medicinal mushrooms. J. Microbiol. Biotechnol. 11: 167–171.
Kim, D. H., B. K. Yang, S. C. Jeong, J. B. Park, S. P. Cho, S. Das, J. W. Yun, and C. H. Song (2001) Production of a hypoglycemic, extracellular polysaccharide from the submerged culture of the mushroom, Phellinus linteus. Biotechnol. Lett. 23: 513–517.
Kim, D. H., B. K. Yang, N. J. Hur, S. Das, J. W. Yun, Y. S. Choi, and C. H. Song (2001) Hypoglycemic effects of mycelia produced from a submerged culture of Phel linus linteus (Berk. et Curt) Teng (Aphyllophoromycetideae) in streptozotocin-induced diabetic rats. Int. J. Med. Mushr. 3: 21–26.
Yang, B. K., D. H. Kim, S. C. Jeong, S. Das, Y. S. Choi, J. S. Shin, S. C. Lee, and C. H. Song (2002) Hypoglycemic effect of a Lentinus edodes exo-polymer produced from a submerged mycelial culture. Biosci. Biotech. Biochem. 66: 937–942.
Hwang, H. J., S. W. Kim, J. M. Lim, J. H. Joo, H. O. Kim, H. M. Kim, and J. W. Yun (2005) Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocininduced diabetic rats. Life Sci. 76: 3069–3080.
Zhang, G., Y. Huang, Y. Bian, J. H. Wong, T. B. Ng, and H. Wang (2006) Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biot. 72: 1152–1156.
Herr, R. R., J. K. Jahnke, and A. D. Argoudelis (1967) The structure of streptozotocin. J. Am. Chem. Soc. 89: 4808–4809.
Schnedl, W. J., S. Ferber, J. H. Johnson, and C. B. Newgard (1994) STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43: 1326–1333.
Like, A. A. and A. Rosani (1976) Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193: 415–417.
Yamagishi, N., K. Nakayama, T. Wakatsuki, and T. Hatayama (2001) Characteristic changes of stress protein expression in streptozotocin induced diabetic rats. Life Sci. 9: 2603–2609.
Kalendra, B., M. Oztruk, M. Tuncdemir, O. Usyal, F. K. Dagistanli, I. Yagenaga, and E. Erek (2002) Renoprotective effect of valsartan and enalapril in STZ-induced diabetes in rats. Acta. Histochem. 104: 123–130.
Stefek, M., N. Tribulova, A, Gajdoski, and A. Gajdosikova (2002) The pyridoindole antioxidant stobadine attenuates histochemical changes in kidney of STZinduced diabetic rats. Acta. Histochem. 104: 413–417.
Imazeki, R. and T. Hongo (1998) Colored illustrations of mushrooms of Japan. pp. 141–142. Hoikusha Press, Osaka, Japan.
Hwang, H. S., S. H. Lee, Y. M. Baek, S. W. Kim, Y. K. Jeong, and J. W. Yun (2008) Production of extracellular polysaccharides by submerged mycelial culture of Laetiporus sulphureus var. miniatus and their insulinotropic properties. Appl. Microbiol. Biot. 78: 419–429.
Junod, A., A. E. Lambert, W. Stauffacher, and A. E. Renold (1969) Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J. Clin. Invest. 48: 2129–2139.
Bolzán, A. D. and M. S. Bianchi (2002) Genotoxicity of streptozotocin. Mutat. Res. 512: 121–134.
Crouch, R., G. Kimsey, D. G. Priest, A. Sarda, and M. G. Buse (1978) Effect of streptozotocin on erythrocyte and retinal superoxide dismutase. Diabetologia 15: 53–57.
Kamtchouing, P., S. D. Sokeng, P. F. Moundipa, P. Watcho, H. B. Jatsa, and D. Lontsi (1998) Protective role of Anacardium occidentale extract against streptozotocin-induced diabetes in rats. J. Ethnopharmacol. 62: 95–99.
Gandy, S. E., M. G. Buse, and R. K. Crouch (1982) Protective role of superoxide dismutase against diabetogenic drugs. J. Clin. Invest. 70: 650–658.
Chakravarthy, B. K., S. Gupta, S. S. Gambhir, and K. D. Gode (1981) The prophylactic action of (-)epicatechin against alloxan-induced diabetes in rats. Life Sci. 29: 2043–2047.
Cam, M. C., W. M. Li, and J. H. McNeill (1997) Partial preservation of pancreatic β-cells by Vanadium: evidence for long-term amelioration of diabetes. Metabolism 46: 769–778.
Stadler, K., M. G. Bonini, S. Dallas, J. J. Jiang, R. Radi, R. P. Mason, and M. B. Kadiiska (2008) Involvement of inducible nitric oxide synthase in hydroxyl radicalmediated lipid peroxidation in streptozotocin-induced diabetes. Free. Radical. Bio. Med. 45: 866–874.
Kaneto, H., J. Fujii, H. G. Seo, K. Suzuki, T. Matsuoka, M. Nakamura, H. Tatsumi, Y. Yamasaki, T. Kamada, and N. Taniguchi (1995) Apoptotic cell death triggered by nitric oxide in pancreatic beta-cells. Diabetes 44: 733–738.
Jang, Y. Y., J. H. Song, Y. K. Shin, E. S. Han, and C. S. Lee (2000) Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharm. Res. 42: 361–371.
Hong, J., M. Bose, J. Ju, J. Ryu, X. Chenm, S. Sang, M. J. Lee, and C. S. Yang (2004) Modulation of arachidonic acid metabolism by curcumin and related β- diketone derivatives: effects on cytosolic phospholipase A2, cyclooxygenases, and 5-lipoxygenase. Carcinogenesis 25: 1671–1679.
Li, X. M. (2007) Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 40: 461–465.
Coskun, O., M. Kanter, A. Korkmaz, and S. Oter (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharm. Res. 51: 117–123.
Friesen, N. T., A. S. Buchau, P. Schott-Ohly, A. Lgssiar, and H. Gleichmann (2004) Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia 47: 676–685.
Oberley, L. W. (1988) Free radicals and diabetes. Free. Radical. Bio. Med. 5: 13–124.
Baynes, J. W. and S. R. Thorpe (1999) Role of oxidative stress in diabetic complication: a new perspective on an old paradigm. Diabetes 48: 1–9.
Robbins, M. J., R. A. Sharp, A. E. Slonium, and I. M. Burr (1980) Protection against streptozotocin-induced diabetes by superoxide dismutase. Diabetologia 18: 55–58.
Sandler, S. and A. Andersson (1982) The partial protective effect of the hydroxyl radical scavenger dimethyl urea on streptozotocin-induced diabetes in the mouse in vivo and in vitro. Diabetologia 23: 374–378.
Li, S. P., G. H. Zhang, Q. Zeng, Z. G. Huang, Y. T. Wang, T. T. X. Dong, and K. W. K. Tsim (2006) Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 13: 428–433.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, H.S., Yun, J.W. Hypoglycemic effect of polysaccharides produced by submerged mycelial culture of Laetiporus sulphureus on streptozotocininduced diabetic rats. Biotechnol Bioproc E 15, 173–181 (2010). https://doi.org/10.1007/s12257-009-0160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0160-6