Abstract
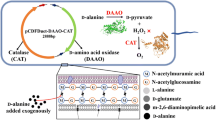
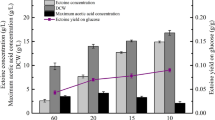
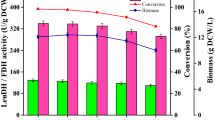
The gene encoding D-amino acid oxidase (DAAO) from Trigonopsis variabilis CBS 4095 has been cloned and expressed in Escherichia coli BL21 (DE3). Unfortunately, it was observed that the host cell was negatively affected by the expressed DAAO, resulting in a remarkable decrease in cell growth. To overcome this problem, we investigated several factors that affect cell growth rate and DAAO production such as addition time of inducer and dissolved oxygen (DO) concentration. The addition time of lactose, which was used as an inducer, and DO concentration appeared to be critical for the cell growth of E. coli BL21 (DE3)/pET-DAAO. A two-stage DO control strategy was developed, in which the DO concentration was controlled above 50% until specific stage of bacterial growth (OD600 30–40) and then downshifted to 30% by changing the agitation speed and aeration rate, and they remained at these rates until the end of fermentation. With this strategy, the maximum DAAO activity and cell growth reached 18.5 U/mL and OD600 81, respectively. By reproducing these optimized conditions in a 12-m3 fermentor, we were able to produce DAAO at a productivity of 19 U/mL with a cell growth of OD600 80.
Similar content being viewed by others
References
Pilone, M. S. (2000) D-Amino acid oxidase: new findings. Cell. Mol. Life Sci. 57: 1732–1747.
Sacchi, S., L. Pollegioni, M. S. Pilone, and C. Rossetti (1998) Determination of D-amino acids using a D-amino acid oxidase biosensor with spectrophotometric and potentiometric detection. Biotechnol. Tech. 12: 149–153.
Cooper, A. J. L., J. Z. Ginos, and A. Meister (1983) Synthesis and properties of the α-keto acids. Chem. Rev. 83: 321–358.
Brodelius, P., B. Hägerdal, and K. Mosbach (1980) Immobilized whole cells of the yeast Trigonopsis variabilis containing D-amino acid oxidase for the production of α-keto acids. Vol. 5. pp. 383–387. In: H. H. Weetal and G. P. Roger (eds.). Enzyme Engineering. Plenum, New York, NY, USA.
Luo, H., Q. Li, H. Yu, and Z. Shen (2004) Construction and application of fusion proteins of D-amino acid oxidase and glutaryl-7-aminocephalosporanic acid acylase for direct bioconversion of cephalosporin C to 7-aminocephalosporanic acid. Biotechnol. Lett. 26: 939–945.
Conlon, H. D., J. Bagai, K. Baker, Y. Q. Shen, B. L. Wong, R. Noiles, and C. W. Rausch (1995) Two-step immobilized enzyme conversion of cephalosporin C to 7-aminocephalosporanic acid. Biotechnol. Bioeng. 46: 510–513.
Pilone, M. S., S. Buto, and L. Pollegioni (1995) A process for bioconversion of cephalosporin C by Rhodotorula gracilis D-amino acid oxidase. Biotechnol. Lett. 17: 199–204.
Szwajcer, D. E., S. Flygare, and K. Mosbach (1991) Stabilization of D-amino acid oxidase from yeast Trigonopsis variabilis used for production of glutaryl-7-aminocephalosporanic acid from cephalosporin C. Appl. Biochem. Biotechnol. 27: 239–250.
Chen, J. T., S. Y. Lin, and H. Tsai (1991) Enzymic and chemical conversions of cephalosporin C to 7-(glut-arylamido)cephalosporanic acid. J. Biotechnol. 19: 203–210.
Matsuda, A., K. Matsuyama, K. Yamamoto, S. Ichikawa, and K. I. Komatsu (1987) Cloning and characterization of the genes for two distinct cephalosporin acylases from a Pseudomonas strain. J. Bacteriol. 169: 5815–5820.
Tsuzuki, K., K. Komatsu, S. Ichikawa, and Y. Shibuya (1989) Enzymatic synthesis of 7-aminocephalosporanic acid. Nippon Nogeikagaku Kaishi 63: 1847–1853.
Volkert, M. R., P. C. Loewen, J. Switala, D. Crowley, and M. Conley (1994) The Δ (argF-lacZ) 205 (U169) deletion greatly enhances resistance to hydrogen peroxide in stationary-phase Escherichia coli. J. Bacteriol. 176: 1297–1302.
Pollegioni, L., L. Caldinelli, G. Molla, S. Sacchi, and M. S. Pilone (2004) Catalytic properties of D-amino acid oxidase in cephalosporin C bioconversion: a comparison between proteins from different sources. Biotechnol. Prog. 20: 467–473.
Golini, P., D. Bianchi, E. Battistel, P. Cesti, and R. Tessinari (1995) Immobilization of D-amino acid oxidase from different yeast: characterization and application in the deamination of cephalosporin C. Enzyme Microb. Technol. 17: 324–329.
Huber, F. M., J. T. Vicenzi, and A. J. Tietz (1992) High-yielding culture conditions for the biosynthesis of D-amino acid oxidase by Trigonopsis variabilis. Biotechnol. Lett. 14: 195–200.
Tishkov, V. I. and S. V. Khoronenkova (2005) D-amino acid oxidase: structure, catalytic mechanism, and practical application. Biochemistry (Mosc). 70: 40–54.
Pilone, M. S., L. D’Anguiro, L. Pollegioni, and S. Buto (1994) Evaluation of D-amino acid oxidase from Rhodotorula gracilis for the production of α-keto acids: a reactor system. Biotechnol. Bioeng. 44:1288–1294.
Pollegioni, L. and M. S. Pilone (1992) Purification of Rhodotorula gracilis D-amino acid oxidase. Protein Expr. Purif. 3: 165–167.
Dib, I., D. Stanzer, and B. Nidetzky (2007) Trigonopsis variabilis D-Amino acid oxidase: control of protein quality and opportunities for biocatalysis through production in Escherichia coli. Appl. Environ. Microbiol. 73:331–333.
Riethorst, W. and A. Reichert (1999) An industrial view on enzymes for the cleavage of cephalosporin C. Chimia. 53: 600–607.
Alonso, J., J. L. Barredo, P. Armisén, B. Díez, F. Salto, J. M. Guisan, J. L. GarcÍa, and E. Cortés (1999) Engineering the D-amino acid oxidase from Trigonopsis variabilis to facilitate its overproduction in Escherichia coli and its downstream processing by tailor-made metal chelate supports. Enzyme Microb. Technol. 25: 88–95.
Lin, L. L., H. R. Chien, W. C. Wang, T. S. Hwang, H. M. Fu, and W. H. Hsu (2000) Expression of Trigonopsis variabilis D-amino acid oxidase gene in Escherichia coli and characterization of its inactive mutants. Enzyme Microb. Technol. 27: 482–491.
Hwang, T. S., H. M. Fu, L. L. Lin, and W. H. Hsu (2000) High-level expression of Trigonopsis variabilis D-amino acid oxidase in Escherichia coli using lactose as inducer. Biotechnol. Lett. 22: 655–658.
Sambrook, J. D. and W. Russel (2001) Molecular Cloning: A Laboratory Manual. 3nd ed. Cold Spring Harbor Laboratory Press, USA.
Szwajcer, D. E., J. R. Miller, S. Kovacevic, and K. Mosbach (1990) Characterization of D-amino acid oxidase with high activity against cephalosporin C from the yeast Trigonopsis variabilis. Biochem. Int. 20: 1169–1178.
Donovan, R. S., C. W. Robinson,and B. R. Glick (1996) Optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J. Ind. Microbiol. 16: 145–154.
Kim, J. E., E. J. Kim, W. J. Rhee, and T. H. Park (2005) Enhanced production of recombinant protein in Escherichia coli using silkworm Hemolymph. Biotechnol. Bioprocess Eng. 10: 353–356.
Yoon, S. H., C. Li, Y. M. Lee, S. H. Lee, S. H. Kim, M. S. Choi, W. T. Seo, J. K. Yang, J. Y. Kim, and S. W. Kim (2005) Production of vanillin from ferulic acid using recombinant strains of Escherichia coli. Biotechnol. Bioprocess Eng. 10: 378–384.
Shin, E. J., S. L. Park, S. J. Jeon, J. W. Lee, Y. T. Kim, Y. H. Kim, and S. W. Nam (2006) Effect of molecular chaperones on the soluble expression of alginate lyase in E. coli. Biotechnol. Bioprocess Eng. 11: 414–419.
Lee, K. W., H. D. Shin, and Y. H. Lee (2002) Extracellular overproduction of β-cyclodextrin glucanotransferase in a recombinant E. coli using secretive expression system. J. Microbiol. Biotechnol. 12: 753–759.
Son, Y. J., K. H. Park, S. Y. Lee, S. J. Oh, C. K. Kim, B. T. Choi, Y. C. Park, and J. H. Seo (2007) Effects of temperature shift strategies on human preproinsulin production in the fed-batch fermentation of recombinant Escherichia coli. Biotechnol. Bioprocess Eng. 12: 556–561.
Lan, J. C., T. C. Ling, G. Hamilton, and A. Lyddiatt (2006) A fermentation strategy for anti-MUC1 C595 diabody expression in recombinant Escherichia coli. Biotechnol. Bioprocess Eng. 11: 425–431.
Kim, M. D., W. J. Lee, K. H. Park, K. H. Rhee, and J. H. Seo (2002) Two-step fed-batch culture of recombinant Escherichia coli for production of Bacillus licheniformis maltogenic amylase. J. Microbiol. Biotechnol. 12: 273–278.
Shiloach, J. and R. Fass (2005) Growing E. coli to high cell density-a historical perspective on method development. Biotechnol. Adv. 23: 345–357.
Makrides, S. C. (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Rev. 60: 512–538.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SJ., Kim, NJ., Shin, CH. et al. Optimization of culture condition for the production of D-amino acid oxidase in a recombinant Escherichia coli . Biotechnol Bioproc E 13, 144–149 (2008). https://doi.org/10.1007/s12257-008-0005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-008-0005-8