Summary
ADCs will likely become another important treatment strategy in advanced, pretreated lung cancer where current treatment options are limited. Both onocogene-directed or biomarker relateted (e.g. EGFR, HER2, MET) and ADCs where no biomarker selection is required (e.g. TROP2, HER3) have either already demonstrated efficacy or under further investigation in NSCLC. Improving the outcomes of patients with metastatic lung cancer is important, thus continued research is necessary to improve understanding of the clinical activity, safety, and optimal sequencing of ADCs in lung cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
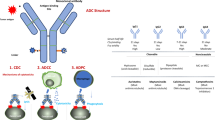
The treatment landscape in lung cancer has changed dramatically over the past few years and now incorporates targeted therapy, immunotherapy, and systemic chemotherapy. Novel antibody–drug conjugates (ADCs) join the list as potential options for lung cancer patients and signify a fundamental shift in the treatment paradigm, with Fam-trastuzumabderuxtecan (T-DXd) already approved and several anticipated approvals on the horizon. ADCs merge the cytotoxic effect of conjugated payload and monoclonal antibody targeted on a specific cancer cell membrane antigen—preferably not expressed on normal tissue, thus with the possibility for more precise treatment and to broaden the therapeutic index of the attached payload—compounds that would otherwise be too toxic for use. Comprising of three key components, ADCs include (a) a monoclonal antibody that binds selectively to an antigen on the tumor cell surface, (b) a cytotoxic drug payload, and (c) a cleavable or noncleavable linker [1].
Components vary between different ADCs, but the mode of action stays the same: the monoclonal antibody binds to its target on the tumor cell, the substance is then internalized by tumor cells, fuses with lysosomes, which leads to the release of the payload. To avoid delivering the cytotoxic payload to nontumor cells, the antibody should preferably recognize targets that are overexpressed on tumor cells. Linkers connecting the antibody to the payload can either be cleavable or noncleavable and, thus, are important for the ADC stability [2, 3].
In the setting of non-small cell lung cancer (NSCLC), there are multiple agents with different targets under development. This review article will summarize the most important findings from recent clinical trials examining ADCs in NSCLC, which demonstrated encouraging antitumor activity and manageable safety profiles. These new drugs provide a panel of opportunities, especially in the second-line setting, where the efficacy of chemotherapy, mainly docetaxel, is limited and toxic side effects are frequent. Improving the outcomes of patients is important to move forward in treating lung cancer; thus, continued research is necessary to improve understanding of the clinical activity, safety, and optimal treatment sequence of ADCs in lung cancer treatment.
ADCs in lung cancer treatment can be categorized into oncogene-directed or biomarker related. Hence, some of these ADCs may be efficacious for patients expressing driver alterations, such as epidermal growth factor receptor (EGFR)- or human epidermal growth factor receptor 2 (HER2)-mutations, hepatocyte growth factor receptor (MET) or carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) overexpression. Other ADCs do not require biomarker selection such as trophoblast cell surface antigen 2 (TROP-2) or human epidermal growth factor receptor 3 (HER3) directed agents.
ADCs in oncogene-driven NSCLC: HER2—Fam-trastuzumab deruxtecan (T-DXd)
HER2 is a transmembrane protein encoded by the erb-b2 receptor tyrosine kinase 2 (ERBB2) gene, which belongs to the ErbB or EGFR family. In NSCLC, HER2 alterations include HER2 gene amplification, HER2 mutations, and HER2 protein overexpression. The frequency of HER2 protein overexpression varies considerably in the literature, although it has been observed in up to 20% of patients with NSCLC and correlates with inferior survival outcomes. Targetable HER2 mutations are rare and occur in approximately 2% of advanced NSCLC cases [4, 5].
Fam-trastuzumab deruxtecan (T-DXd) is an ADC that contains the humanized anti-HER2 monoclonal antibody trastuzumab connected to the topoisomerase inhibitor deruxtecan (DXd) using a protease-cleavable peptide linker [6].
It is currently the only ADC approved in Europe for the treatment in second line of adult patients with unresectable or metastatic NSCLC harboring activating HER2 mutations. Results from the multicenter randomized phase 2 DESTINY-Lung02 trial, which evaluated the safety and efficacy of T‑DXd in patients with metastatic NSCLC harboring an activating HER2 mutation and who had previously received at least one line of platinum-based chemotherapy, supported the approval. Two different doses (5.4 mg/kg every 3 weeks or 6.4 mg/kg) were evaluated.
Regarding baseline characteristics, most patients in the 5.4 mg/kg arm and the 6.4 mg/kg arm had HER2 mutations predominantly in the kinase. Slightly more than half of patients in both arms were never smokers. At baseline, 34.3% and 44.0% of patients had stable central nervous system (CNS) metastases. The median number of prior regimens received for both arms was 2, with a range of 1 to 12. All patients enrolled in the trial had prior exposure to platinum-based chemotherapy and the majority (73.5% and 78.0%) previously had received immunotherapy [7].
If given at a dose of 5.4 mg/kg every 3 weeks (n = 102), it elicited a confirmed objective response rate (ORR) of 49.0% (95% confidence interval [CI] 39.0–59.1%) by blinded independent central review (BICR) in this population. Complete response (CR) was seen in 1% of patients, 48% had a partial response (PR), and 44.1% had stable disease (SD), whereas 3.9% experienced disease progression (PD) and 2.9% were not evaluable for response. The disease control rate (DCR) was 93.1% (95% CI 86.4–97.2%). The median duration of response (DOR) was 16.8 months (95% CI 6.4–not evaluable) and the median time to initial response (TTIR) was 1.8 months (range 1.2–7.0) [7].
Responses with the ADC were reported in both treatment arms, and irrespective of number or type of prior systemic treatment and baseline CNS metastases. Moreover, patients in both arms experienced tumor reduction from baseline, which was sustained over time.
The toxicity profile of T‑DXd at both doses was in line with previously reported adverse events with this ADC, including nausea, decreased red and white blood cell count and interstitial lung disease (ILD). Treatment-emergent adverse effects of grade 3 or higher occurred in 38.6% of those who received the ADC at 5.4 mg/kg, and the most common included neutropenia (18.8%) and anemia (10.9%). Moreover, 12.9% of patients in this group were diagnosed with ILD or pneumonitis determined to be related to treatment, the majority grade 1 or 2 (10.9%), with one grade 3 and one grade 5 event [7].
In summary, T‑DXd demonstrated deep and durable responses at both treatment doses. The safety profile was acceptable and generally manageable at both doses and favored the 5.4 mg/kg dose. Lower incidence of drug-related treatment-emergent adverse events (TEAEs), drug discontinuations, dose reductions, and drug interruptions as well as lower rates of drug-related ILD were observed with the 5.4 mg/kg dose. These results are very exciting, as this is the first targeted drug to effectively treat patients with this alteration and introduces ADCs as a novel class of drugs into the field of lung cancer treatment.
EGFR mutant NSCLC—patritumab deruxtecan (HER3-DXd)
Patients with EGFR-mutated NSCLC treated with a tyrosine kinase inhibitor (TKI) often experience recurrence due to resistance mechanisms. In this setting few effective treatment options remain. The HER3-directed ADC patritumab deruxtecan (HER3-DXd) demonstrated encouraging results for patients with locally advanced or metastatic, EGFR-mutated NSCLC who previously received at least two systemic therapies according to findings from the multicenter, open-label phase 2 HERTHENA-Lung01 trial. Patients were initially randomly assigned to receive intravenous patritumab deruxtecan every 3 weeks at a fixed dose of 5.6 mg/kg (n = 225) or via an uptitration dosing schedule (n = 50). Patients enrolled in the study were heavily pretreated with a median of three prior lines of therapy (range 1–11)—the majority had received a third-generation EGFR TKI (93%) and 40% immunotherapy. At baseline, most patients were female (58.7%), never smokers (64.0%), younger than 65 years of age (53.8%), had EGFR exon 19 deletions (63.1%), a history of CNS metastasis (51%), and had an ECOG performance status (PS) of 1 (66.2%) [8].
Data were presented during the IASLC 2023 World Conference on Lung Cancer and demonstrated an ORR of 29.8% (95% CI 23.9–36.2%) among patients with EGFR-mutated locally advanced or metastatic NSCLC following disease progression after an EGFR-directed TKI and platinum-based chemotherapy (n = 225). In addition, the disease control rate (DCR) was 73.8% (95% CI 67.5–79.4%), the median duration of response (DOR) was 6.4 months (95% CI 4.9–7.8), and the median progression-free survival (PFS) was 5.5 months (95% CI 5.1–5.9). The confirmed intracranial ORR in patients with brain metastasis at baseline who did not undergo prior radiotherapy (n = 30) was 33.3% (95% CI 17.3–52.8%). Clinically meaningful responses were observed among various mechanisms of EGFR TKI resistance including EGFR-dependent (ORR 32.4%) and EGFR-independent pathways (ORR 27.2%) as well as those without any identified resistance mechanism (ORR 27.3%) [8]. At a median follow-up of 18.9 months, the median OS was 11.9 months (95% CI 11.2–13.19).
Patritumab deruxtecan displayed a manageable and tolerable toxicity profile. However, most patients experienced at least one any-grade TEAE (99.6%). TEAEs were associated with treatment discontinuation (7.1%), dose reduction (21.3%), and dose interruption (40.4%). Grade 3 or higher TEAEs occurred at a rate of 64.9%. Treatment-related TEAEs occurred in most patients (95.6%), events of grade 3 or higher severity (45.3%), and serious TEAEs (15.1%).
Interstitial lung disease occurred sparingly (5.3%) and was reported at grade 1 (0.4%), grade 2 (3.6%), grade 3 (0.9%), and grade 5 (0.4%) severity. Other common grade 1 or 2 TEAEs included nausea (63%), thrombocytopenia (44%), decreased appetite (39%), and constipation (34%). Grade 3 or higher TEAEs included thrombocytopenia (21%), neutropenia (19%), anemia (14%), and leukopenia (10%) [8].
These data are very encouraging in heavily pretreated patients with advanced EGFRm NSCLC as efficacy was observed across a broad range of HER3 expression and various mechanisms of EGFR TKI resistance. Furthermore, antitumor activity was seen in patients with brain metastases, which underlines the importance of patritumab deruxtecan as a treatment option for patients with EGFRm NSCLC after TKI failure.
Ongoing trials with patritumab deruxtecan include a phase 3 trial vs platinum-based chemotherapy in patients with EGFR-mutated NSCLC after progression on third-generation EGFR TKI therapy (HERTHENA-Lung02) and a phase 1 trial in combination with osimertinib in patients with EGFRm NSCLC after progression on first-line osimertinib or in previously untreated patients.
MET overexpression—telisotuzumab vedotin (Teliso-V)
The tyrosine kinase receptor c‑Met is commonly overexpressed in many different solid tumor types and is present in approximately one quarter of patients with NSCLC. Patients with c‑Met overexpression have poor prognosis and opposed to patients with MET exon 14 mutations currently no approved targeted treatment options are available [9,10,11]. Telisotuzumab vedotin (Teliso-V) is a first-in-class, c‑Met protein directed ADC, composed of anti-c-Met humanized mAb ABT-700 attached to monomethyl auristatin E (MMAE) via a valine-citrulline linker. It was investigated for patients with MET overexpression, but also in the resistant setting for patients with EGFR mutant NSCLC who had received a prior TKI [12, 13].
According to findings from an interim analysis of the single-arm, phase 2 LUMIOSITY/M14-239 trial, Teliso‑V produced meaningful response rates in patients with c‑Met protein overexpressed, EGFR wild-type, advanced or metastatic nonsquamous NSCLC. Patients whose disease has progressed on, or after, platinum-based chemotherapy received 1.9 mg/kg of intravenous Teliso‑V every 2 weeks as second- or third-line therapy.
In the nonsquamous population c‑Met overexpression was defined as intermediate (IHC 3+, ≥ 25 to < 50%) or high (IHC 3+, ≥ 50%); 75% or greater expression (IHC 1+) was required in the squamous population.
The ORR was 35% in patients with c‑Met high expression and 23% in patients with intermediate expression. With a median duration of response (DOR) of 9 months and 7.2 months in the c‑Met high and intermediate populations, respectively, Teliso‑V seems to be a promising agent for this patient cohort. The median overall survival (OS) was similar in both groups at 14.6 and 14.2 months. Treatment-related adverse effects were manageable and tolerable, with no new safety signals and a consistent toxicity profile as reported previously. The most common any-grade AEs were peripheral sensory neuropathy (25.0%), nausea (22.1%), and hypoalbuminemia (20.6%). Grade 5 AEs potentially related to Teliso‑V occurred in 2 patients with squamous disease from sudden death and pneumonitis [14].
Teliso‑V is also under evaluation versus docetaxel in the randomized phase 3 TeliMET NSCLC-01 trial in patients with previously treated c‑Met overexpressing, EGFR wild-type nonsquamous NSCLC and in combination with osimertinib (Tagrisso) in the phase 1 M14-237 trial, which will broaden our understanding of Teliso-Vs therapeutic potential.
ADCs without biomarker selection: trophoblast cell surface antigen (TROP2)
TROP2 is a transmembrane glycoprotein calcium signal transducer that mediates cell migration and anchorage-independent growth, and is expressed across many epithelial tumors [15]. It has been associated with poor OS and disease-free survival (DFS) in several types of solid tumors [16]. In lung cancer, Trop2 overexpression was observed in up to 64% of adenocarcinoma and up to 75% of squamous cell carcinoma NSCLC and is associated with reduced survival [17].
Two TROP-2-directed ADCs are currently under development: sacituzumab govitecan and datopotamab deruxtecan (Dato-DXd).
Sacituzumab govitecan is a first-in-class anti-Trop2 ADC, which consists of humanized anti-Trop2 monoclonal antibody sacituzumab linked to the topoisomerase I inhibitor SN-38 by a hydrolysable cleavable linker. Dato-DXd is a TROP2-directed ADC consisting of a humanized anti-TROP2 IgG 1 monoclonal antibody covalently linked to a highly potent topoisomerase I inhibitor payload via a plasma-stable, tumor-selective, tetrapeptide-based cleavable linker [18]. Both ADCs have demonstrated efficacy in the second-line setting and in highly pretreated patients [19].
At the 2023 ESMO Congress, data from the phase 3 TROPION-LUNG01 study, investigating Dato-DXd versus docetaxel in the second-line setting, were presented. Patients treated with Dato-DXd (n = 299) experienced an increased PFS of 4.4 months (95% CI 4.2–5.6) vs 3.7 months as compared to docetaxel. The PFS advantage was driven by patients with nonsquamous histology with an PFS of 5.6 months (95% CI 4.4–7.0) in the Dato-DXd arm (n = 229) vs 3.7 months (95% CI 2.9–4.2) in the docetaxel arm (n = 232; HR 0.63; 95% CI 0.51–0.78) [20]. The interim OS findings also favor Dato-DXd, and the trial is continuing to the final analysis.
In the phase 1 TROPION-PanTumor01 study, Dato-DXd showed promising efficacy in patients with actionable genomic alterations [21]. TROPION-Lung05, a phase 2, single-arm study is currently evaluating Dato-DXd in patients with advanced or metastatic NSCLC with actionable genomic alterations that progressed on or after targeted therapy and platinum-based chemotherapy. In this heavily pretreated group of patients, Dato-DXd achieved a median ORR of 43.6% (95% CI 32.4–55.3%) for those with EGFR mutations (n = 78) and 23.5% (95% CI 10.7–41.2%) for those with ALK rearrangements (n = 34), with a median DOR of 7 months. The median PFS was 5.8 months (95% CI 5.4–8.3) and 4.3 months (95% CI 2.6–6.9), respectively [22]. Specific toxicities of grade 3 or higher found with Dato-DXd include stomatitis (11%), ocular toxicities (2%) and ILD (1%).
Data from the phase 3 EVOKE-01 trial of sacituzumab govitecan versus docetaxel are awaited in order to be able to better compare the two agents [23]. From smaller datasets in the first-line setting in combination with immunotherapy, similar efficacy data have been demonstrated for the two substances.
Initial results from the ongoing phase 2 EVOKE-2 trial demonstrated that the addition of sacituzumab govitecan to pembrolizumab produced high response rates in the frontline setting for patients with advanced NSCLC. Across all patients, the ORR with the combination regimen was 56%, with a DCR of 82% and responses were durable [24, 25].
Toxicity profiles of the two TROP‑2 directed ADCs differ—sacituzumab govitecan resulted in more cytopenias but less mucositis. Hence, proactive, specific management of the specific side effects will likely become highly important.
Conclusion, challenges, and future perspectives
ADCs open up an entirely new treatment strategy for patients with advanced lung cancer and currently very limited treatment options. The armamentarium will be expanded to include this new class of drugs, as individual treatment substances or in combination with immunotherapy and/or chemotherapy. Currently, additional novel targets under further investigations in advanced NSCLC include CEACAM5, NECTIN4, tissue factor, mesothelin, and LIV1. Due to their comparably larger size, blood–brain barrier penetration and, thus, intracranial efficacy might be of concern. It is assumed that large molecules such as monoclonal antibodies and ADCs do not cross the blood–brain barrier, so that combination strategies with smaller molecules and radiation might be necessary. However, analyses from the DESTINY-Lung 01 and DESTINY-Lung02 study demonstrated that T‑DXd achieved intracranial response rates between 30 and 50% with a reduction in brain lesion size from baseline of approximately 80%, indeed suggesting a considerable and rather unexpected cerebral efficacy of these ADCs.
Currently, ADCs are investigated in advanced NSCLC and preferably later therapy lines only. Until today, there are no registered trials of ADCs in early stage NSCLC, although the previous evolution of immunotherapy and targeted therapies suggest that this may change rapidly in the next few years.
New challenges will occur with these new promising types of therapies and physicians and patients will have to face them; furthermore, different therapy-associated side effects may require different therapeutic strategies than under currently used anticancer drugs.
The optimal position in the therapy sequence still needs to be established.
The optimal strategy for patient selection for these treatments is not yet known. Hence, ongoing research in biomarkers to better predict treatment responses is urgently needed.
References
Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 22. März 2022;7(1):93.
Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9(1):33–46.
Nolting B. Linker technologies for antibody-drug conjugates. Methods Mol Biol. 2013;1045:71–100.
Hirsch FR, Varella-Garcia M, Franklin WA, Veve R, Chen L, Helfrich B, u. a. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br J Cancer. 6. Mai 2002;86(9):1449–56.
Liu L, Shao X, Gao W, Bai J, Wang R, Huang P, et al. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol. 2010;5(12):1922–32.
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, u. a. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T‑DM1. Clin Cancer Res. 15. Oktober 2016;22(20):5097–108.
Goto K, Goto Y, Kubo T, Ninomiya K, Kim SW, Planchard D, u. a. Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J Clin Oncol. 1. November 2023;41(31):4852–63.
Yu HA, Goto Y, Hayashi H, Felip E, Chih-Hsin Yang J, Reck M, u. a. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J Clin Oncol. 10. Dezember 2023;41(35):5363–75.
Park S, Choi YL, Sung CO, An J, Seo J, Ahn MJ, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histol Histopathol. 2012;27(2):197–207.
Liang H, Wang M. MET Oncogene in Non-Small Cell Lung Cancer: Mechanism of MET Dysregulation and Agents Targeting the HGF/c-Met Axis. Onco Targets Ther. 2020;13:2491–510.
Guo B, Cen H, Tan X, Liu W, Ke Q. Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. Plos One. 2014;9(6):e99399.
Camidge DR, Morgensztern D, Heist RS, Barve M, Vokes E, Goldman JW, u. a. Phase I Study of 2‑ or 3‑Week Dosing of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c‑Met, Monotherapy in Patients with Advanced Non-Small Cell Lung Carcinoma. Clin Cancer Res. 1. November 2021;27(21):5781–92.
Camidge DR, Barlesi F, Goldman JW, Morgensztern D, Heist R, Vokes E, u. a. Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c‑Met Protein-Expressing Non-Small-Cell Lung Cancer. J Clin Oncol. 10. Februar 2023;41(5):1105–15.
AbbVie announces positive topline results from phase 2 LUMINOSITY trial evaluating telisotuzumab-vedotin (Teliso-V) for patients with previously treated non-small cell lung cancer (NSCLC). News release. AbbVie. November 29. 2023. https://news.abbvie.com/2023-11-29-AbbVie-Announces-Positive-Topline-Results-from-Phase-2-LUMINOSITY-Trial-Evaluating-Telisotuzumab-Vedotin-Teliso-V-for-Patients-with-Previously-Treated-Non-Small-Cell-Lung-Cancer-NSCLC. Accessed 7 Feb 2024.
Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop‑2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 8. September 2015;6(26):22496–512.
Pak MG, Shin DH, Lee CH, Lee MK. Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J Surg Oncol. 6. April 2012;10:53.
Jiang A, Gao X, Zhang D, Zhang L, Lu H. Expression and clinical significance of the Trop‑2 gene in advanced non-small cell lung carcinoma. Oncol Lett. 2013;6(2):375–80.
Parisi C, Mahjoubi L, Gazzah A, Barlesi F. TROP‑2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2023;118:102572.
Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32(6):746–56.
Ahn MJ, Lisberg A, Paz-Ares L, Cornelissen R, Girard N, Pons-Tostivint E, et al. LBA12 Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): Results of the randomized phase III study TROPION-Lung01. Ann Oncol. 2023;34:1305–6.
Shimizu T, Sands J, Yoh K, Spira A, Garon EB, Kitazono S, u. a. First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2‑Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non-Small-Cell Lung Cancer: TROPION-PanTumor01. J Clin Oncol. 10. Oktober 2023;41(29):4678–87.
Paz-Ares L, Ahn MJ, Lisberg AE, Kitazono S, Cho BC, Blumenschein G, et al. 1314MO TROPION-Lung05: Datopotamab deruxtecan (Dato-DXd) in previously treated non-small cell lung cancer (NSCLC) with actionable genomic alterations (AGAs). Ann Oncol. 2023;34:755–6.
Garassino MC, Reznick D, Liu SY, Reinmuth N, Girard N, De Marinis F, u. a. EVOKE-01: A phase 3 study of sacituzumab govitecan (SG) versus docetaxel in patients with non-small cell lung cancer (NSCLC) progressing on or after platinum-based chemotherapy and checkpoint inhibitors. JCO. 1. Juni 2022;40(16_suppl):TPS9149–TPS9149.
Garon EB, Liu SV, Owen SP, Reck M, Neal JW, Vicente D, u. a. EVOKE-02: A phase 2 study of sacituzumab govitecan (SG) plus pembrolizumab (pembro) with or without platinum chemotherapy in first-line metastatic non-small cell lung cancer (NSCLC). JCO. 1. Juni 2022;40(16_suppl):TPS9146–TPS9146.
Cho BC, Cobo Dols M, Cabanillas RR, et al. Sacituzumab Govitecan + Pembrolizumab in 1L Metastatic Non-Small Cell Lung Cancer: Preliminary Results of the EVOKE. 2023. 02 Study.
Funding
Open access funding provided by Johannes Kepler University Linz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. E. Wass, D. Lang, A. Horner and B. Lamprecht declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.