Summary
Antibody-drug conjugates (ADCs) are a new class of monoclonal antibodies with the characteritic to specifically target tumor cells and to deliver a cytotoxic drug directly to the target cell thereby minimising side effects and improving the therapeutic index. In recent years, many new ADCs have been approved and are established therapeutic tools in lymphoma treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
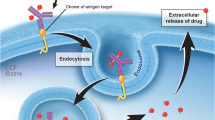
Chemotherapy represents an essential therapeutic option for the treatment of various forms of lymphoid malignancies. However, due to their nonspecific mode of action, these agents are usually associated with dose-limiting toxicities and ultimately drug resistance. With the development of monoclonal antibodies (mAb), a novel technology called antibody–drug conjugate (ADC), comprising a mAb linked to a small cytotoxic molecule via a covalent linker (Fig. 1), has emerged as a novel class of promising immunotherapy, presumably because of their high potency combined with high tumor selectivity. Once attached to the corresponding cell-surface antigen of tumor cells, the ADC is internalized, the cytotoxic payload is released, causing cell apoptosis.
Brentuximab vedotin
Already in use for several years is the anti-CD30 antibody brentuximab vedotin (BV; Adcetris®, Takeda Pharma A/S, Vallensbaek Strand, Denmark), coupled to the cytostatic monomethyl auristatin‑E (MMAE), which inhibits the polymerization of tubulin and, thus, the formation of microtubules during mitosis. Thus, the cell remains in G2/M phase and finally goes into apoptosis. Currently, brentuximab is approved for adult patients with Hodgkin’s disease in clinical stage III and IV with previously untreated Hodgkin’s lymphoma (HL) in combination with doxorubicin, vinblastine, and dacarbazine (AVD) [1], the so-called ECHELON‑1 study, or as maintenance/consolidation in patients with relapsed CD30-positive HL at increased risk of relapse or progression following autologous stem cell transplantation (ASCT), the AETHERA trial [2]. In the ECHELON‑1 study, 664 patients were assigned to receive Adcetris + AVD and 670 to receive ABVD. In this study, the 6‑year overall survival (OS) estimates were 93.9% in the A + AVD group and 89.4% in the ABVD group. Also, the progression-free survival (PFS) was significantly longer with A + AVD than with ABVD. Moreover, fewer patients in the A + AVD group than in the ABVD group received subsequent therapy, including transplantation, and fewer second cancers were reported with A + AVD [1]. In the randomized SWOG 1826 study, 6 cycles of nivolumab (N)-AVD were compared to 6 cycles of BV-AVD in > 940 patients in newly diagnosed stage III and IV Hodgkin lymphoma. After a median follow-up of 12.1 months, N‑AVD significantly improved 1‑year PFS (94%) compared to BV-AVD (86%). A subgroup analysis in patients aged > 60 years (n = 97 patients) confirmed that N‑AVD markedly improved PFS over BV-AVD in older cHL patients with 1‑year PFS of 93% vs 64% in favor of the N‑AVD arm. Thus, in this elderly population N-AVD will be the new first-line standard in advanced stage cHL [3].
In the phase 3 AETHERA trial, BV as a consolidative therapy for classical Hodgkin lymphoma after ASCT showed a significantly improved progression-free survival (PFS) vs placebo at 5‑year follow-up. The 5‑year PFS was 59% with BV vs 41% with placebo. In particular, patients with ≥ 2 risk factors benefitted from the BV treatment and experienced significantly higher PFS at 5 years and a significantly delayed time to second subsequent therapy. Peripheral neuropathy, the most common adverse event in patients receiving BV, continued to improve in the majority of patients [2]. BV as monotherapy is also licensed to treat patients with relapsed or refractory HL based on the pivotal phase 2 trial of BV in patients with relapsed/refractory (R/R) Hodgkin lymphoma after failed hematopoietic autologous stem cell transplantation. At 5 years, the overall patient population (n = 102) had an estimated OS rate of 41% and a PFS rate of 22% [4]. In addition, 38% of patients achieving complete remission (CR) on BV have remained in remission for > 5 years and may be cured, some of them without a consolidative allogeneic stem cell transplant [4]. Eagerly awaited are the results of the HD 21 study of the GHSG intended to improve the outcome of the HD 18 study by remodeling the toxic eBEACOPP regimen with BV [5]. In this study treatment with brentuximab vedotin plus the chemotherapy combination of etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone (BrECADD) was found to be non-inferior to escalated BEACOPP (Bleomycin, Etoposid, Adrimaycin, Cyclophophamid, Vincristin, Procarbacin and Prednison) in patients with advanced classical Hodgkin lymphoma. At a median follow-up of 40 months the 3‑year PFS in the intention-to-treat (ITT) population was 94.9% in the BrECADD arm vs 92.3% in the escBEACOPP arm. These survival results demonstrate that individualized treatment with PET2-guided BrECADD is an effective therapy for advanced-stage cHL and sets a new benchmark for the primary treatment in advanced cHL [6].
Most important BrECADD was significantly less toxic with regard to peripheral neuropathy and showed a more favorable preservation of fertility profile [5]. A recent update at the 46-month follow-up demonstrates that the recovery of follicle-stimulating hormone following BrECADD was higher in both women (age 18–39) and men. Approximately one in ten patients reported pregnancies in HD21 and pregnancy rates were twofold higher in partners of men who received BrECADD and were also higher in women [7]. Red blood cell and platelet transfusions were also reduced in the BrECADD arm.
A further example of reducing toxicity is the BREACH trial, where the problematic use of toxic bleomycin by adding brentuximab to the combination doxorubicin, vinblastine, and dacarbazine (AVD) in the first-line setting in high-risk Hodgkin patients resulted in an improvement in PET negativity rate (82% vs. 75%) and 2‑year PFS (97% vs. 93%). A recently published phase III study also confirmed this benefit in terms of OS (94% vs. 89%) after a median follow-up of 73 months [8]. In the relapsed setting, BV is also frequently and successfully administered in combination with various agents as bendamustine [9], or checkpoint inhibitors like nivolumab [10] or pembrolizumab [11].
BV is also used in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) in adult patients with previously untreated systemic anaplastic large cell lymphoma (sALCL) [12] and as single substance in the treatment of patients with relapsed or refractory sALCL [13]. In the randomized ECHELON-2 study, 452 patients were randomized (1:1) to six or eight cycles of Adcetris + CHP or CHOP in the frontline treatment of patients with systemic anaplastic large cell lymphoma or other CD30-positive PTCL. The 5‑year PFS rates were 51.4% with A + CHP versus 43.0% with CHOP alone and the 5‑year OS rates were 70.1% with versus 61.0%. Interestingly, among patients who relapsed and subsequently received brentuximab vedotin, the objective response rate was still 59% with brentuximab vedotin retreatment after A + CHP [12].
Camidanlumab tesirine
Camidanlumab tesirine for the therapy of R/R Hodgkin lymphoma was assessed in a phase 2, open-label, single-arm study. Results from 117 heavily pretreated patients demonstrated that the substance is effective in patients who had received three or more prior lines of systemic therapies including prior treatment with brentuximab vedotin and PD-1 inhibitors. The objective response rate was 70.1% consisting of 33.3% CRs, 36.8% PRs, and 17.9% stable disease. The median duration of response was 13.7 months and the median PFS was 9.1 months in the treated population. Before approval, a confirmatory randomized phase 3 study is planned [14].
Polatuzumab vedotin
Currently approved for the treatment of relapsed/refractory diffuse large B‑cell lymphoma (DLBCL) is polatuzumab vedotin (Polivy®, Genentech, South San Francisco, CA, USA), a monoclonal antibody targeting CD79b, a component of the B‑cell receptor. The antibody is also linked to the cytostatic drug MMAE. A phase Ib/II trial enrolled DLBCL patients with at least one prior therapy who were unsuitable for transplantation or already had transplantation as prior therapy [15]. The three-drug combination polatuzumab vedotin with bendamustine and rituximab was evaluated against bendamustine rituximab. After a median follow-up of 22.3 months, there was a significantly improved PFS (hazard ratio [HR] 0.36; p < 0.01) for the triple combination. OS was also statistically significantly superior (HR 0.42; p = 0.002), with a median duration of response of 4.1 versus 10.3 months. In a post hoc analysis, this benefit was confirmed for all clinical and biological subgroups. Regarding adverse events, there was a higher rate of hematologic adverse events in the Pola-Benda‑R arm, with a similar rate of grade 3–4 infections. The occurrence of peripheral polyneuropathy was reported in just under half of patients, and it also led to discontinuation of therapy in 2 patients [15].
The POLARIX trial [16] evaluated first-line use of the combination of polatuzumab and rituximab along with CHP (cyclophosphamide, doxorubicin, prednisolone) compared with the current gold standard rituximab–CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) in over 800 patients. A 27% risk reduction in progression, relapse or death (HR 0.73; p = 0.02) was achieved in the experimental arm. After a median follow-up of 28 months, 24-month PFS was superior at 77 to 70%, and no difference was observed in OS. Overall response was excellent, with high CR rates in both treatment arms (87% vs. 83%). 22.5% in the pola-R-CHP group and 30.3% in the R‑CHOP group had received at least one subsequent line of lymphoma therapy. Consequently, the percentage of patients receiving radiotherapy, systemic therapy, stem cell transplantation, or CAR T‑cell therapy was lower in the pola-R-CHP group. Subgroup analyses revealed that the highest benefit with pola-R-CHP was observed in patients older than 60 years, in patients with an IPI score between 3 and 5, and in patients with the activated B‑cell-like (ABC) subtype of DLBCL. Toxicity was comparable and although the incidence of febrile neutropenia was higher among patients receiving pola-R-CHP this did not translate into a higher incidence of infection or treatment discontinuation [16].
Polatuzumab vedotin is currently investigated together with glofitamab [17] or mosunetuzumab [18] in patients with relapsed/refractory DLBCL and is also being compared (Pola and mosunetuzumab) to rituximab GemOx [19].
Loncastuximab tesirine
Another ADC is loncastuximab tesirine (Zylonta®, ADC Therapeutics, Epalinges, Switzerland), which was approved by the US Food and Drug Administration (FDA) in April 2021 and by the European Medicines Agency (EMA) in December 2022. It consists of an antibody that binds to the lymphocyte antigen CD19 and contains the toxin tesirine. The multicenter, single-arm phase II LOTIS‑2 [20] trial demonstrated good response rates in 145 pretreated DLBCL patients (median of 3, or at least 2 prior therapies). Loncastuximab was administered intravenously every 3 weeks, and 70 patients achieved remission, 35 of whom achieved complete remission. The median duration of response was 10.3 months, and 13.4 months for patients in CR. The median PFS was 4.9 months, and the median OS was 9.9 months. In addition, it was shown that despite targeted CD19 therapy with loncastuximab, subsequent CAR T‑cell therapy was also successful in individual patients. Documented side effects were hematologic toxicities including febrile neutropenia, as well as edema, pleural and pericardial effusions, and laboratory changes in liver enzymes. In an updated efficacy and safety analyses from LOTIS‑2 with a median follow-up of 7.8 months, 70 of 145 (48.3%) patients achieved an overall response. Thirty-six (24.8%) patients achieved CR, of which 16 (44%) and 11 (31%) were event-free for ≥ 1 year and ≥ 2 years, respectively [21]. Hence, loncastuximab continued to demonstrate durable and long-term responses with a manageable safety profile, particularly in patients with CR.
Inotuzumab ozogamicin
Inotuzumab ozogamicin (Besponsa®, Pfizer, New York, NY, USA) targets its antibody to CD22, an epitope found on 90% of B‑ALL cells, and induces apoptosis of the labeled cells via calicheamicin. Inotuzumab is indicated as monotherapy for the treatment of adults with relapsed or refractory CD22-positive B‑precursor ALL and for patients with Philadelphia chromosome-positive (Ph+) relapsed or refractory B‑precursor ALL after an unsuccessful treatment with at least one tyrosine kinase inhibitor (TKI). In a phase 3 trial, 326 patients were randomly assigned to receive either inotuzumab ozogamicin or standard intensive chemotherapy [22]. Inotuzumab resulted in a significant improvement in CR rate (80.7% vs. 29.4%; p < 0.001) and a longer duration of remission (4.6 months vs. 3.1 months; p = 0.03) over standard chemotherapy. The PFS was significantly longer in the inotuzumab ozogamicin group (median, 5.0 months vs. 1.8 months) and the median overall survival was 7.7 months versus 6.7 months. However, the high proportion of liver toxicities up to the occurrence of sinusoidal obstruction syndrome, which was documented in 13% of patients, should be taken into account [22]. In the final report with a long-term survival follow-up the median OS was superior in favor of inotuzumab with 2‑year OS rates of 22.8 and 10.0%, respectively [23]. The predictors of OS with inotuzumab were the best minimal residual disease status, duration of first remission, achievement of CR/CRi, and a consolidative allogeneic stem cell transplantation. More inotuzumab patients (48.2% vs 22.2%) proceeded directly to HSCT after achieving complete remission [22]. For patients with Ph+ disease, CR/CRi rates, MRD negativity, and 12-month PFS were all improved with inotuzumab although there was no OS benefit [24].
Belantamab mafodotin
Belantamab mafodotin (Blenrep®, GlaxoSmithKline, London, UK) is an antibody–drug conjugate that combines a monoclonal antibody, which binds specifically to B‑cell maturation antigen (BCMA) with the cytotoxic agent maleimidocaproyl monomethyl auristatin F. Marketing authorization through the European Union was issued for as monotherapy for the treatment of relapsed/refractory multiple myeloma (MM) with at least four prior therapies including one proteasome inhibitor (PI), one immunomodulatory agent (IMiD), and an anti-CD38 monoclonal antibody (mAb). However, in the DREAMM‑3 a randomized study comparing belantamab mafodotin to oral pomalidomide and dexamethasone, no statistically improved progression-free survival compared to the standard of care arm was achieved and, thus, the conditional authorization was not granted by EMA [25].
Moxetumomab pasudotox
Moxetumomab pasudotox (Lumoxiti®, Medimmune, Gaithersburg, MD, USA) is a first-in-class recombinant CD22 antibody-directed cytotoxin (pseudomonas exotoxin) approved for the treatment of adult patients with relapsed/refractory hairy cell leukemia (HCL) with at least two prior lines of systemic treatment including purine nucleoside analogues. Eighty patients with a median number of three prior systemic lines were treated with moxetumomab pasudotox. The overall CR rate was 41.3 and 81.6% patients who achieved a CR also achieved a MRD-negative status. The median duration of hematologic remission from CR was 62.8 months and median progression-free survival was 41.5 months. However, in July 2023, due to economic reasons, moxetumomab pasudotox was permanently discontinued from the market [26]
Take home message
The use of antibody–drug conjugates (ADCs) in hematologic neoplasms has led to a significant improvement in outcome in recent years. In addition to numerous already established ADCs, other promising compounds are being investigated in clinical trials. Relevant is the often unfamiliar side effect profile that the treating physician faces with the use of ADCs.
References
Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A, Ramchandren R, Friedberg JW, Advani R, Hutchings M, Evens AM, Smolewski P, Savage KJ, Bartlett NL, Eom HS, Abramson JS, Dong C, Campana F, Fenton K, Puhlmann M, Straus DJ. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma.ECHELON‑1 Study Group. N Engl J Med. 2022;28;387(4):310–20. Jul.
Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Viviani S, Bachanova V, Sureda A, McClendon T, Lee C, Lisano J, Sweetenham J. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;20;132(25:2639–42.
Rutherford SC, Hongli L, Herrera AF, et al. Nivolumab-AVD is better tolerated and improves progression-free survival compared to Bv-AVD in older patients (aged ≥60 years) with advanced stage Hodgkin lymphoma enrolled on SWOG S1826. Presented at ASH 2023. December. San Diego, CA Abstract. 2023;181:9–12.
Chen R, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;.
Borchmann P, Moccia A, Grell R, et al. Treatment related morbidity in patients with classical hodgkin lymphoma: results of the ongoing, randomized phase III HD21 trial by the German Hodgkin Study Group. Blood. 2022;140((suppl 1):771–3.
Borchmann P et al. ICML 2023 Abstract LBA4. https://lymphome.de/fileadmin/Media/leistungen/LymphomKompetenzKompakt/2023-ICML/Borchmann/Borchmann-ICML2023-SH.pdf.
Ferdinandus J, et al. ASH 2023 Abstract #4437. https://ash.confex.com/ash/2023/webprogram/Paper188449.html.
Fornecker LM, Lazarovici J, Aurer I, Casasnovas RO, Gac AC, Bonnet C, Bouabdallah K, Feugier P, Specht L, Molina L, Touati M, Borel C, Stamatoullas A, Nicolas-Virelizier E, Pascal L, Lugtenburg P, Di Renzo N, Vander Borght T, Traverse-Glehen A, Dartigues P, Hutchings M, Versari A, Meignan M, Federico M, André M; LYSA-FIL-EORTC Intergroup. Brentuximab Vedotin Plus AVD for First-Line Treatment of Early-Stage Unfavorable Hodgkin Lymphoma (BREACH): A Multicenter, Open-Label, Randomized, Phase II Trial. J Clin Oncol. 2022
LaCasce AS, et al. Brentuximab. a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood: vedotin plus bendamustine; 2018. Vol 132, Number 1.
Ranjana H. Advani. et al., Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3‑year study results. Blood, 2021 Vol 138, NUMBER 6.
Massaro F, et al. Brentuximab Vedotin and Pembrolizumab Combination in Patients with Relapsed/Refractory Hodgkin Lymphoma: A Single-Centre Retrospective Analysis. Cancers. 2022;14:982.
Horwitz s. et al. The ECHELON‑2 Trial. 5‑year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T‑cell lymphomaAnnals of Oncology. Volume. 2022;33(3):288–98.
Barbara Pro, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. BLOOD, 21; 2017, Vol 130, Number 25.
Carmelo Carlo-Stella, et al. Camidanlumab Tesirine: updated efficacy and safety in an open-label, multicenter, phase 2 study of patients with relapsed or refractory classical hodgkin lymphoma (R/R CHL). EHA. 2022;201.
Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B‑Cell Lymphoma. J Clin Oncol. 2020 Jan 10;38(2):155–165. https://doi.org/10.1200/JCO.19.00172.
Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, Herbaux C, Burke JM, Matasar M, Rai S, Izutsu K, Mehta-Shah N, Oberic L, Chauchet A, Jurczak W, Song Y, Greil R, Mykhalska L, Bergua-Burgués JM, Cheung MC, Pinto A, Shin HJ, Hapgood G, Munhoz E, Abrisqueta P, Gau JP, Hirata J, Jiang Y, Yan M, Lee C, Flowers CR, Salles G. Polatuzumab Vedotin in Previously Untreated Diffuse Large B‑Cell Lymphoma. N Engl J Med. 2021;14.
Hutchings M, et al. Glofitamab plus polatuzumab vedotin demonstrates durable responses and a manageable safety profile in patients with relapsed/refractory diffuse large B‑cell lymphoma. ICML. 2023.
Adam J Olszewski, et al. Mosunetuzumab with polatuzumab vedotin is effective and has a manageable safety profile in patients aged < 65 and ≥ 65 years with relapsed/refractory DLBCL and ≥ 1 prior therapy: subgroup analysis of a Phase Ib/II study. Poster Presentation at the 64th ASH Annual Meeting P1630.
Jason Westin, et al. SUNMO: A Phase III Trial Evaluating the Efficacy and Safety of Mosunetuzumab in Combination with Polatuzumab Vedotin versus Rituximab in Combination with Gemcitabine plus Oxaliplatin in Patients with Relapsed or Refractory Aggressive B‑cell Non-Hodgkin Lymphoma. Poster Presentation at the 64th ASH Annual Meeting P1637.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, Kahl BS, Radford J, Solh M, Stathis A, Zinzani PL, Havenith K, Feingold J, He S, Qin Y, Ungar D, Zhang X, Carlo-Stella C. Loncastuximab tesirine in relapsed or refractory diffuse large B‑cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):790–800. Jun.
Caimi PF, et al. Loncastuximab tesirine in relapsed/refractory diffuse large B‑cell lymphoma: long-term efficacy and safety from the phase 2 LOTIS-2 study. Haematologica. 2023;31.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gökbuget N, O’Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E, Advani AS. Inotuzumab Ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Kantarjian HM, et al. Inotuzumab Ozogamicin Versus Standard of Care in Relapsed or Refractory Acute Lymphoblastic Leukemia: Final Report and Long-Term Survival Follow-Up From the Randomized, Phase 3 INO-VATE Study. Cancer. 2019;125:2474–87.
Stock W, Martinelli G, Stelljes M, DeAngelo DJ, Gokbuget N, Advani AS, et al. Efficacy of inotuzumab ozogamicin in patients with Philadelphia chromosome-positive relapsed/refractory acute lymphoblastic leukemia. Cancer. 2021;127(6):905–13.
Dimopoulos MA, et al. Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): a phase 3, open-label, randomised study. Lancet Haematol. 2023 Oct;10(10):e801–e812.
Kreitman RJ, et al. Moxetumomab Pasudotox-Tdfk in Heavily Pretreated Patients with Relapsed/Refractory Hairy Cell Leukemia (HCL): Long-Term Follow-up from the Pivotal Phase 3 Trial. Blood. 2019;134(Suppl. 1 Nov 13).
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P. Neumeister and K.T. Prochazka declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumeister, P., Prochazka, K.T. Antibody–drug conjugates in the treatment of lymphoid neoplasms. memo 17, 135–139 (2024). https://doi.org/10.1007/s12254-024-00965-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-024-00965-x