Summary
Background
Seminal vesicle metastasis from colon adenocarcinoma is very rare, with only two case reports in the literature. Also, positron emission tomography/computed tomography (PET/CT) imaging in the diagnosis of seminal vesicle tumors seems to have a promising role in its detection, but there have been few reports about its use, in part due to the rarity of the disease.
Case presentation
We report the case of a 64-year-old male patient with colon adenocarcinoma who at 6 years after the diagnosis presented with metastasis to the seminal vesicles, which responded remarkably to chemotherapy, but 2 years later had a relapse at the seminal vesicles. Imaging with PET/CT was helpful for the diagnosis of this case.
Conclusion
Our case is unique, as there are no previous reports in the literature on the relapse of metastatic colon adenocarcinoma in the seminal vesicles. Imaging with PET/CT showed compromise of the seminal vesicles in our case; hence, this imaging technique seems to have a promising role in detecting metastatic seminal vesicle tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Seminal vesicle metastasis from colon adenocarcinoma is very rare, with only two case reports in the literature [1, 2]. We report a case of colon adenocarcinoma metastatic to the seminal vesicles discovered 6 years after the initial diagnosis, which responded remarkably to chemotherapy. However, 2 years later the patient had a relapse, with metastasis to the seminal vesicles; this led to hydronephrosis, which became infected and shortly after that the patient died. This is the first case report documenting relapse of a metastatic disease of colon adenocarcinoma in the seminal vesicles and highlights the utility of positron emission tomography/computed tomography (PET/CT) in the diagnosis of seminal vesicle tumors, which allowed us to confirm the metastatic tumor.
Case report
The patient was a 64-year-old man with a history of transverse colon adenocarcinoma (KRAS and NRAS wild type), diagnosed during emergency laparotomy and colectomy due to intestinal obstruction. Operative findings showed a mass in the transverse colon (5 × 5 × 1.5 cm), while biopsies confirmed serosa, lymphovascular, and perineural invasion, and 13 of 20 lymph nodes were involved. Extension studies performed before commencing chemotherapy revealed a hepatic lesion with a metastatic appearance, measuring 19 mm in segment VIII. Hence, the tumor was classified as pT4apN2bM1G2 and the patient received six cycles of FOLFOX‑6 chemotherapy with liver metastasectomy (segment VIII). As part of the follow-up, an abdominal magnetic resonance imaging (MRI) study performed 14 months later revealed a hepatic lesion in segment III suggestive of metastasis. Thus, in view of a recurrence, liver metastasectomy (segment III) was performed, followed by three cycles of FOLFOX‑7 chemotherapy.
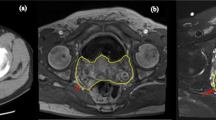
In the subsequent follow-up 6 years later, MRI showed a nodular hypervascular neoplastic-looking mass in the right seminal vesicle measuring 23 × 15 mm, with compromise of the mesorectal fascia (Fig. 1) and a metastatic infiltrative lesion in the fifth lumbar vertebrae. Based on these findings, it was decided to perform 18F-FDG PET-CT, which revealed a hypermetabolic nodule in the right lobe of the seminal vesicle with a tumor appearance with 4.7 SUVs (standardized uptake value), and hypermetabolic bilateral iliac nodes (left common iliac lymph nodes 4.4 SUV, 10 mm; and in the right external lymph nodes, 2.5 SUVs); hence, a relapse was suspected (Fig. 2). The CEA levels were without significant alterations. Due to the infrequent presentation of such lesions, a transrectal targeted ultrasound-guided biopsy with cognitive fusion was performed. Ultrasound findings showed a left dilated seminal vesicle without suspicious lesions; the right seminal vesicle was enlarged with a hypoechoic image with calcifications and solid-looking areas inside, normal prostatic vesicle angles, and a prostate volume of 25.6 cc (Fig. 3). The biopsy consisted of four core samples for the seminal vesicles, two cores for each side, without complications. It is worth noting that at the patient denied any urological symptom or pain.
Tissue biopsy showed a poorly differentiated adenocarcinoma, and immunohistochemical markers were positive in the tumoral glands for CK-20 and CDX2 and negative for CK‑7, with positive control of CK‑7 in the normal glands of the seminal vesicle. The prostate-specific antigen (PSA) immunohistochemical marker results were negative. The previously described immunohistochemical profile was compatible with a colorectal primary origin in a metastatic fashion (Fig. 4). Additionally, a computed tomography scan of the chest revealed multiple nodules in both lungs, with the largest ones measuring 7 mm in the upper right lobe and 8 mm in the upper left lobe; findings were suggestive of neoplastic disease.
Considering the confirmation of relapse in the right seminal vesicle, regional lymph nodes, bone, and lungs, second-line chemotherapy was indicated with six cycles of FOLFIRI plus panitumumab. Chemotherapy was received with adequate tolerance, and continued maintenance therapy was administered with 5‑fluorouracil and panitumumab. Two months after completing the sixth cycle of FOLFIRI plus panitumumab, there was resolution of the seminal vesicle lesions (Fig. 5) but a partial response in the bone lesions. Six months later (during the seventh cycle of 5‑fluorouracil and panitumumab maintenance therapy), bone scintigraphy, and lumbar spine MRI revealed disease progression due to the appearance of new metastatic infiltrative lesions in the third and fourth lumbar vertebrae (the lesion in the fifth lumbar vertebrae persisted), and CEA levels without significant alterations; hence, chemotherapy with a regimen of FOLFIRI and cetuximab was started. However, after the eighth session of this chemotherapy regimen, the patient presented with hematologic (anemia) and dermatologic toxicity (generalized skin rash); therefore, the chemotherapy regimen was changed to bevacizumab plus capecitabine. During the 17th session of this chemotherapy regimen, the patient complained of severe lumbar pain that was difficult to control with analgesics, and given that the bone lesions on the follow-up bone scintigraphy and lumbar spine MRI did not show any signs of regression, intensity-modulated radiation therapy (IMRT) of the lumbar spine with palliative intent at 2000 cGy in fractions of 400 cGy per day was started.
The patient completed this treatment with adequate tolerance and improvement of symptoms. During follow-up at 3 months after the completion of radiotherapy, the patient was referred for fever and increased pain, and was sent to the emergency department where he was diagnosed with a urinary tract infection A urinary tract ultrasound showed bilateral hydronephrosis (predominantly right) due to a hypoechoic nodule of oval morphology and well-defined contours in the right seminal vesicle (Fig. 6); hence an MRI of the pelvis was performed, which confirmed metastatic involvement of the right seminal vesicle (Fig. 7) with no signs of regression of the bone lesions. Therefore, the patient required nephrostomy and systemic chemotherapy was discontinued. The patient completed an antibiotic regimen of meropenem for 7 days and voriconazole for 14 days, as in the urine culture Trichosporon asahii, Pseudomona aeruginosa, and Enterococcus faecalis were isolated, and since the patient had adequate symptomatic control after the completion of the antibiotics he was discharged with the nephrostomy. Due to pelvic relapse, the plan was a new cycle of IMRT at the site of relapse with palliative intent at 3900 cGy in fractions of 300 cGy per day, with resumption of chemotherapy with FOLFOX once the infection was controlled.
Urinary tract ultrasound. a An oval-shaped, well-defined hypoechoic nodule is identified in the right seminal vesicle, with approximate dimensions of 15 × 22 mm. b, c Bilateral pyelocaliectasis is observed, with the anteroposterior diameter of the right renal pelvis measuring 11.4 mm (b) and the left measuring 28 mm (c)
Two weeks after hospital discharge, the patient presented again with his family to the emergency department because he was initially aggressive and had ripped off the nephrostomy. Hours later he became somnolent, the proposed antineoplastic management had not yet been initiated. Initially, the patient had a Glasgow Coma Scale score of 11/15; when alert, there was the impression of pain. The patient’s vital signs were normal, and initial paraclinical tests confirmed a relapse of the urinary tract infection. The patient presented with rapid clinical deterioration as his state of consciousness worsened and he became hypotensive and tachycardic. He died shortly after.
Discussion
Malignant transformation of the seminal vesicles is very uncommon. Tumors involving the seminal vesicles are mainly secondary tumors originating from adjacent organs. The tumors that most commonly compromise the seminal vesicles are prostate adenocarcinoma, urothelial carcinoma, and rectal adenocarcinoma [3, 4]. Seminal vesicle metastasis from the transverse colon is very rare, with only two case reports in the literature describing this uncommon pattern of metastasis of primary colon adenocarcinoma [1, 2].
Primary tumors arising in the seminal vesicles are unusual, with adenocarcinoma being the most common form. Given its rarity, diagnosis is delayed and is unlikely to be made at an early stage due to the absence of symptoms, and when patients become symptomatic, it is highly suggestive of advanced disease; symptoms include obstructive uropathy, hematuria, hematospermia, dysuria, painful defecation, or pelvic and perineal pain [5, 6].
On imaging, some findings suggestive of seminal vesicle tumors include loss of normal seminal vesicle architecture, seminal vesicle enlargement or wall thickening with a low-signal-intensity mass on T2-weighted images, obliteration of the angle between the prostate and seminal vesicles, and appearance of a soft-tissue mass in the retrovesical region with or without prostatic or ureteral obstruction [1, 7].
To date, there are no specific clinical or imaging features in conventional or nuclear imaging that enable the differentiation of a primary from a secondary form of malignancy; thus, an accurate diagnosis remains based on immunohistochemistry [8].
The histological pattern of seminal vesicle adenocarcinoma is defined as moderate-to-poorly differentiated adenocarcinoma with at least focal papillary architecture and tubular structures. The tumor cell cytoplasm may show a clear cell or hobnail morphology. Immunohistochemically, primary seminal vesicle adenocarcinoma is characterized by CK7-positive, CK20-negative, CA-125-positive, PSA/PAP-negative immunophenotypes that distinguish it from other tumors that metastasize to the seminal vesicles (Table 1; [9, 10]).
Recently, PET combined with CT has emerged as a powerful tool for the evaluation of many cancers. The radiotracer most commonly used for PET in oncology is 2‑[18F] fluoro-2-deoxy-d-glucose (18F-FDG), a glucose analog that is preferentially taken up and trapped inside metabolic hyperactive tumor cells; hence, areas with increased 18F-FDG intake are suspected to be malignant. However, the use of 18F-FDG in urology is limited because of its physiological excretion through the urinary system; thus, PET imaging is not used as frequently as it is in other medical specialties. 18F-FDG has proven to be accurate, not only for urologic tumors but also for detecting metastatic disease. 18F-FDG is, therefore, commonly used in clinical practice as a diagnostic method during the follow-up of oncology patients with a serologic relapse in which conventional imaging methods do not show conclusive results. No SUV standards have been reported in the literature for seminal vesicle tumors, partly because of the rarity of the condition [11, 12].
Normally, the diagnostic algorithm for primary seminal vesicle carcinoma is the performance of a CT scan upon clinical suspicion, and depending on the results, the mass is biopsied for pathology studies [13]. There is only one case report in which the initial diagnosis was made with 18F-FDG, and described that the radiotracer accumulated only in the seminal vesicles; therefore, the authors argued that 18F-FDG could offer a faster and more accurate preoperative differential diagnosis of a primary tumor from a secondary tumor. For example, differentiating whether the mass of the seminal vesicles is due to invasion from the prostate or originates directly from the seminal vesicles is challenging with CT, but 18F-FDG accumulation solely on seminal vesicles confirms the diagnosis of primary seminal vesicle carcinoma. The advantages of PET/CT over CT as an initial study cannot be established with only one case report [14].
Regarding the diagnosis of seminal vesicle metastasis, it has been reported that the initial study is CT, and if it does not show results that explain the relapse of the oncology patient, PET/CT is performed [15]. In reports in which PET/CT was required during the diagnostic process [3, 4, 14, 16, 17], this technique showed a compromise of the seminal vesicles, as in our case. There are only two reports in which PET/CT was used to diagnose metastasis of seminal vesicles from the colon [3, 4]. To the best of our knowledge, there is no case report of a diagnosis of secondary seminal vesicle adenocarcinoma for which PET/CT was the initial diagnostic test.
With few reports on the use of PET/CT in the diagnosis of seminal vesicle tumors, partly because of the rarity of the disease, this imaging technique seems to have a promising role in detecting metastatic and primary seminal vesicle tumors.
The treatment of seminal vesicle adenocarcinoma usually involves surgical resection of the tumor and chemotherapy [18]. In cases of secondary seminal vesicle adenocarcinoma, the chemotherapeutic regimen is generally based on the primary metastatic tumor, and usually treatment of metastatic colon adenocarcinoma relies on systemic immunotherapy, radiotherapy, or surgical resection. Because in our case the primary tumor was a stage IV colon adenocarcinoma (distant metastasis), a FOLFIRI regimen, leucovorin/5-fluoracil + irinotecan (choosing 5‑fluorouracil) plus a targeted drug, which in our case was the monoclonal antibody against the epidermal growth factor receptor (anti-EGFR) panitumumab, was chosen. In cases of relapse of secondary seminal vesicle adenocarcinoma, which has not been described for metastatic colon adenocarcinoma, management should be based again on the primary metastatic tumor, and thus a regimen of FOLFOX (folinic acid, 5‑fluorouracil, and oxaliplatin) plus IMRT was chosen; however, in our patient it was never started because of infectious compromise [19].
Surgical excision can have a role in treatment if the patient’s metastasis is confined to one site or if it is an oligometastatic disease; furthermore, patient age, life expectancy, and functional status must be taken into account. Laparoscopic excision of the seminal vesicle due to metastatic disease from colon adenocarcinoma has been described in one case report, but the patient only had metastasis to the seminal vesicles. By contrast, surgical excision was not feasible in our patient because he did not have oligometastatic disease (progression to the right seminal vesicle, regional lymph nodes, bone, and lungs), and by the time of relapse, he had a poor functional status [1, 20].
Radiosurgery plays an important role in the management of bone metastases, as in the case of our patient. The primary goal of radiosurgery in bone metastases is to provide effective pain relief and improve quality of life for patients. This is achieved by targeting and destroying tumor cells in the affected bone, thereby reducing tumor burden and relieving pain. In addition to pain relief, radiosurgery can also help prevent fractures and stabilize weakened bones. By delivering high doses of radiation to tumor sites, it can cause tumor shrinkage and promote bone healing. This can be particularly beneficial in cases where bone metastases have compromised the structural integrity of the bone and increased the risk of fractures. Radiosurgery is typically used in conjunction with other treatment approaches, such as surgery or systemic therapies, depending on the individual patient’s needs and the characteristics of the metastatic disease. The use of radiosurgery after chemo-targeted therapy depends on the type and stage of cancer, as well as the patient’s overall health. In some cases, radiosurgery may be used to treat any remaining cancer cells after chemo-targeted therapy. In other cases, it may be used in combination with chemo-targeted therapy to improve the effectiveness of treatment. This may be because the targeted therapy can help sensitize tumor cells to radiation, making them more susceptible to the effects of radiosurgery [21,22,23].
Given that metastasis to the seminal vesicles is rare, standard treatment guidelines are not available; hence, a multidisciplinary team approach is recommended.
Conclusion
We report a unique case of seminal vesicle metastasis from colon adenocarcinoma with recurrence, which has not been documented before. Positron emission tomography/computed tomography (PET/CT) imaging played a crucial role in the diagnosis of our patient, highlighting the potential of PET/CT as an imaging technique for detecting metastatic seminal vesicle tumors. Further studies are needed to better characterize its utility.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 18F-FDG:
-
2-[18F] fluoro-2-deoxy-d-glucose
- CEA:
-
Carcinoembryonic antigen
- cGy:
-
Centigray
- CK:
-
Cytokeratin
- IMRT:
-
Intensity-modulated radiation therapy
- MRI:
-
Magnetic resonance imaging
- PET/CT:
-
Positron emission tomography and computed tomography
- SUVs:
-
Standardized uptake value
References
Hsu YL, Lin IC, Tung CL. 18F-FDG PET/CT of Seminal Vesicle Metastasis from Ascending Colon Adenocarcinoma. Clin Nucl Med. 2017;42(2):138–9.
Atici SD, Ugurlu L, Aydin C. Isolated metastasis of left seminal vesicle due to colon adenocarcinoma: An unusual pattern of metastasis. J Coll Physicians Surg Pakistan. 2021;31(6):752–3.
Kim B, Kawashima A, Ryu JA, Takahashi N, Hartman RP, King BF. Imaging of the seminal vesicle and vas deferens. Radiographics. 2009;29(4):1105–21.
Lopez-Beltran A, Menendez CL, Montironi R, Cheng L. The Prostate and Seminal Vesicles. In: Lopez-Beltran A, Menendez CL, Montironi R, Cheng L, editors Rare Tumors and Tumor-like Conditions in Urological Pathology. Springer International Publishing: cham, 2015. pp 195-310 https://doi.org/10.1007/978-3-319-10253-5_3.
Reddy MN, Verma S. Lesions of the Seminal Vesicles and their MRI Characteristics. J Clin Imaging Sci. 2014;4(4):61.
Egevad L, Ehrnström R, Håkansson U, Grabe M. (2007) Primary Seminal Vesicle Carcinoma Detected at Transurethral Resection of Prostate. Urology. 69(4):778.e11–778.e13.
Sala E, Akin O, Moskowitz CS, Eisenberg HF, Kuroiwa K, Ishill NM, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: Diagnostic accuracy and multivariate feature analysis. Radiology. 2006;238(3):929–37.
Bhat A, Banerjee I, Kryvenko ON, Satyanarayana R. Primary seminal vesicle adenocarcinoma: A lethal yet cryptic malignancy with review of literature. BMJ Case Rep. 2019;12(12):1–6.
Bhardwaj N, Rastogi P, Attri VS, Bora GS, Gorsi U. Primary seminal vesicle adenocarcinoma: A case report of rare entity and discussion of its differential diagnosis using immunohistochemical approach for the core biopsy specimen. Andrologia. 2020;52(3):1–5.
Ormsby AH, Haskell R, Jones D, Goldblum JR. Primary seminal vesicle carcinoma: An immunohistochemical analysis of four cases. Mod Pathol. 2000;13(1):46–51.
Kitajima K, Yamamoto S, Fukushima K, Minamimoto R, Kamai T, Jadvar H. Update on advances in molecular PET in urological oncology. Jpn J Radiol. 2016;34(7):470–85.
Høilund-Carlsen PF, Poulsen MH, Petersen H, Hess S, Lund L. FDG in urologic malignancies. PET Clin. 2014;9(4):457–68.
Lal H, Yadav P, Jena R, Jain M. Metastatic primary seminal vesicle adenocarcinoma: Management of a rare tumour with multiagent chemotherapy and hormonal therapy. BMJ Case Rep. 2017;2017.
Mizuno N, Fujikawa N, Murakami T, Suzuki T, Ikeda I. A case of primary seminal vesicle cancer detected by FDG-PET/CT. Nihon Hinyokika Gakkai Zasshi. 2012;103(6):704–7.
Jadvar H, Colletti PM, Delgado-Bolton R, Esposito G, Krause BJ, Iagaru AH, et al. Appropriate use criteria for 18F-FDG PET/CT in restaging and treatment response assessment of malignant disease. J Nucl Med. 2017;58(12):2026–37.
Cheng XB, Lu ZQ, Lam W, Yiu MK, Li JS. Solitary seminal vesicle metastasis from ileal adenocarcinoma presenting with hematospermia: A case report. World J Clin Cases. 2021;9(23):6775–80.
Sollini M, Silvotti M, Casali M, Giovanardi F, Zadro A, Froio A, et al. The Role of Imaging in the Diagnosis of Recurrence of Primary Seminal Vesicle Adenocarcinoma. World J Mens Health. 2014;32(1):61.
Katafigiotis I, Sfoungaristos S, Duvdevani M, Mitsos P, Roumelioti E, Stravodimos K, et al. Primary adenocarcinoma of the seminal vesicles. A review of the literature. Arch Ital Di Urol E Androl. 2016;88(1):47–51.
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, NCCN Clinical Practice Guidelines in Oncology. Jnccn J Natl Compr Cancer Netw. 2021;19(3):329–59.
Aponte-Colon DA, Xu JT, Sorcini A. Seminal vesicle mass: An unusual site for metastatic hepatocellular carcinoma after orthotopic liver transplant. Urol Case Rep. 2021;9;40:101938.
Spencer KL, van der Velden JM, Wong E, Seravalli E, Sahgal A, Chow E, Verlaan JJ, Verkooijen HM, van der Linden YM. Systematic Review of the Role of Stereotactic Radiotherapy for Bone Metastases. J Natl Cancer Inst. 2019;1;111(10):1023–32.
De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;11;8(15):25691–9.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9.
Funding
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Contributions
J. Arenas Hoyos: contributed to the conception of the work in addition to the acquisition of data, participated in the writing of the paper and supervision; A.F. Gutierrez Rojas: contributed to the conception of the work in addition to the acquisition of data, participated in the writing of the paper and supervision; J. Serrano Giraldo: participated in the writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J. Arenas Hoyos, J. Serrano Giraldo and A.F. Gutierrez Rojas declare that they have no competing interests.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case. For images or other information within the manuscript which identify patients, consent was obtained from them and/or their legal guardians.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arenas Hoyos, J., Serrano Giraldo, J. & Gutierrez Rojas, A.F. Seminal vesicle metastasis from transverse colon adenocarcinoma: a unique case report. memo (2024). https://doi.org/10.1007/s12254-023-00951-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12254-023-00951-9