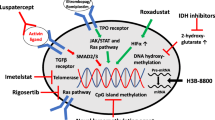
Summary
This article intends to summarize and comment on some of the highlights regarding myelodysplastic syndrome (MDS) presented at the 2022 American Society of Hematology (ASH) annual meeting. Many abstracts dealt with the validation of the two new classifications and the International Prognostic Scoring System–Molecular (IPSS-M) being among the most intensively discussed topics in the community. Moreover, for the first time, real-world data on luspatercept were presented. Long-term data from the MEDALIST trial showed which patients benefit most from therapy with luspatercept, adding important information for the use of this substance. However, except for the phase III trial Sintra-REV, practice-changing clinical reports were sparse, although earlier trials in both higher-and lower-risk MDS reported on promising agents currently in clinical development that will hopefully improve the future management of MDS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the 2022 hybrid ASH annual meeting, several interesting studies in the field were reported. To us, three main fields seem to be the most relevant and will be detailed in this article: (1) the topic of ‘prognostication and classification’, (2) management of lower-risk patients including real-world data on luspatercept (Bristol Myers Squibb), long-term follow-up data of the MEDALIST trial, and the final analysis of the phase III Sintra-REV study, and (3) new approaches for higher-risk patients. When appropriate, reference is made how to integrate the results into clinical practice.
Results
Classification and prognostication
At ASH 2021, the long-awaited Molecular International Prognostic Scoring System (IPSS-M) was presented and full publication immediately followed [1]. In 2022 this was the topic of numerous abstracts dealing with the validation and prognostic ability of this score in real-world cohorts among others from Europe [2] and the US [3]. It can be concluded that the IPSS‑M improves the prognostic accuracy for progression-free survival (PFS) and overall survival (OS) when compared to the IPSS‑R (R: revised), allowing optimized therapeutic decision making. According to one study, the IPSS‑M also improved posttransplant outcome prediction (survival and prediction of relapse [2]). The latter observation highlights that the IPSS‑M is potentially a better tool for hematopoietic stem cell transplantation (HSCT) candidate selection. In a broader context, this observation targets the following question: what to do with patients who are classified as lower-risk myelodysplastic syndrome (LR-MDS) according to the IPSS‑R but higher-risk MDS (HR-MDS) according to the IPSS‑M. In the original publication, the majority of reclassified patients were up-staged, but management of up-staged patients remains unclear [1]. Although evidence and survival data from prospective trials are currently lacking, one may consider more intensive therapy regimens for up-staged patients, including potentially curative treatment strategies with induction therapy and consolidating HSCT. Limitations of the IPSS‑M from a global view include lack of resources and highly complex analyses.
Furthermore, as two new classifications for MDS (WHO 5th edition [4] and ICC classification [5]) were introduced in 2022, validations were presented and the pros and cons of each classification were critically discussed [6]. Among many overlaps, the blast cut-off is one main difference between the two classifications and gives rise to discussion. To overcome this controversial point, Haferlach et al. presented data to exclude blast counting and categorize MDS solely based on genetic abnormalities [7]. Nine biologically distinct disease groups with substantial differences in OS could be defined by solely considering the karyotype and molecular data. The known favorable outcome of SF3B1 mutations and isolated del(5q)-mutated patients together with the poor outcome of bi-allelic TP53-mutated patients could be confirmed. In addition, complex karyotype and RUNX1 mutation were associated with poor outcome. Within patients carrying spliceosome mutations, RUNX1 and ASXL1 define distinct subgroups, harboring higher progression tendency. Overall, this discussion highlights that a detailed genetic work-up is becoming more and more important, although morphologic analysis can currently not be eliminated from any diagnostic work-up of suspected MDS and MDS/AML. However, the question of the optimal blast limit still remains a point of intense discussion.
Lower-risk MDS
In the lower-risk setting (LR-MDS), the focus was clearly set on detailed data on luspartacept (Lus) in the setting for transfusion-dependent LR-MDS with ring sideroblasts (RS) and/or SF3B1 mutations. Final data of the phase III Sintra-REV trial and emerging therapies such as Imetelstat (Geron) were also reported.
Luspatercept
First, MEDALIST long-term follow-up data were provided and highlighted that long-term responders had the following profile: patients were younger, had a lower transfusion burden, lower serum ferritin and serum erythropoietin levels at baseline, were more likely to have mutated SF3B1, and less likely to have received previous ESA in the 6 months prior to study entry [8]. Even though the trial was not powered for OS/PFS analysis, luspatercept responders displayed a superior OS, whereas no difference in PFS was detected compared to the nonresponders [9].
In addition, for the first time real-world data for luspatercept confirmed MEDALIST data with respect to overall response rate of approximately 40%. Of interest, presented data also showed that transfusion independence can be achieved in patients with previous HMA or lenalidomide (Len) failure, although at lower response rates (30% HMA failure patients vs. 50% for HMA naïve patients and 33% for Len failure patients vs. 43% Len naïve patients) [10]. This study was performed in a cohort from the US, where HMA is a common treatment after ESA failure, which is usually not applied as off-label therapy in Europe.
Another real-world data set consisting of 76 patients receiving luspatercept confirmed the high response rate and beneficial safety profile [11]. According to this work, the most common reason for luspatercept discontinuation was progression to HR-MDS, but none of the patients discontinued treatment due to adverse events. However, the majority of patients discontinued their therapy at the starting dose (1 mg/kg). One should be aware that dose titration can further improve response rates, and dose escalation (up to 1.73 mg/kg) is effective, especially in highly transfusion-dependent patients. Thus, dose escalation should be performed in clinical practice before treatment discontinuation.
What all these studies on luspatercept have in common is that transfusion burden is a major predictive factor for response. The interim analysis from the phase III COMMANDS trial was recently presented at the EHA conference. Luspatercept was shown to be superior to ESA in ESA-naïve, transfusion-dependent LR-MDS patients in the first-line setting, especially among MDS patients with ring sideroblats ± SF3B1 mutations. This highlights that early use of luspatercept is even more effective and will probably redesign the first-line treatment strategy [12].
del5(q) patients
The final results of the Sintra-REV, a phase III multicenter trial in low-risk MDS-del(5q) patients with transfusion-independent anemia—evaluating the use of lenalidomide vs. placebo —were presented [13]. Thereby low-dose lenalidomide (5 mg), if started before transfusion dependence, significantly prolonged time to transfusion dependency (69.8% risk reduction compared with placebo). Also response rates, both erythroid and cytogenetic, were better in the lenalidomide-treated cohort (77.8% and 94.1%, respectively). However, early use of lenalidomide did not translate into improved OS. A separate poster abstract highlighted that lenalidomide treatment is safe with regard to molecular evolution, including TP53 mutation [14]. Based on evidence from other work [15] which raised concerns that lenalidomide can put selection pressure on TP53-mutated clones, the latter observation needs further attention, as patient numbers are small and the observation periods may not be long enough for the detection of a potentially harmful effect in patients with pre-existing TP53-mutated subclones. One major limitation to this study is that quality-of-life assessment was not reported. This would be of high interest as anemia and time to transfusion could be improved but OS was not affected. Full publication of this work is eagerly awaited, as this may introduce lenalidomide even before transfusion independence in del(5q)-mutated patients.
Other LR-MDS patients
There are also promising data for patients with LR-MDS from the phase II IMerge trial investigating the telomerase inhibitor Imetelstat [16]: Platzbecker et al. presented the characteristics of patients who were non-del(5q), refractory to ESA and lenalidomide, HMA naïve, and had continued transfusion independence for more than one year while on Imetelstat. Transfusion independency for > 1 year was achieved in 29% of patients (median duration 92.4 weeks), thus, highlighting the high potency of this drug. In the phase III trial (NCT02598661), which was also presented at this year’s EHA meeting, the primary endpoint was met (8 week transfusion independence), making this another promising treatment option for LR-MDS patients [17].
Higher-risk MDS
Only few relevant data were presented for patients with high-risk HR-MDS. Unfortunately, also after the ASH 2022 meeting the question about how to move the landscape forward for this particularly difficult-to-treat patient population remains unanswered. While there are sometimes discussions about triplet therapies, one must admit that we still do not have an approved duplet (e.g., Venetoclax/Azacitidine) therapy for HR-MDS. Results for a potential combination therapy were presented: the data of the STIMULUS-MDS1, which is a phase II trial investigating the combination of HMA (decitabine/azacitidine) plus a TIM‑3 antibody (Sabatolimab®, Novartis) vs. HMA plus placebo [18], were highly disappointing. After promising results from the phase Ib study [19], the current phase II study could not meet its primary endpoint (PFS, complete remission rates). There was also no difference in OS. Only a slight benefit of the combination therapy was reported for the overall response rate (49.2 vs. 37.1%) with durable length especially among the patients achieving complete remission. The phase III STIMULUS-MDS2 (NCT04266301) study with the primary endpoint of OS has completed recruitment and will provide further information on the effect of Sabatolimab plus HMA in high-risk MDS.
Based on the phase III ASCERTAIN trial, the oral drug cedazuridine/decitabine (Taiho Oncology) was approved in the USA in 2020 for the treatment of intermediate‑1, intermediate‑2, and high-risk MDS [20]. In contrast, in Europe approval for MDS is still pending. At this ASH, a post hoc analysis of the phase III ASCERTAIN trial data, looking at TP53-mutated patients, was presented [21, 22]. Overall, 44 patients (35%) were TP53 mutated, thereof 68% had a monoallelic mutation and 32% had a biallelic/multihit status. Although TP53 mutation was associated with poor prognosis when compared to TP53wt patients (median OS 13 vs. 29.9 months), treatment with cedazuridine/decitabine revealed comparable OS data when compared to survival data for HMA treatment (9.5 months [23]). Future trials investigating this drug will be needed; thus, this drug may also serve as a backbone for combination trials in the future.
Conclusion and outlook
In addition to state-of-the-art morphological analyses, careful molecular work-up (including conventional cytogenetics/fluorescence in situ hybridization [FISH] and next generation sequencing [NGS]) should be part of every myelodysplastic syndrome (MDS) work-up. Although a better understanding of classification and prognostication is relevant for treatment decisions and the patient’s overall management, treatment of MDS (in particular of higher-risk MDS) remains a huge challenge. In lower-risk disease, luspatercept will be a future standard of care as first-line therapy. Moreover, earlier treatment of del(5q) MDS even before transfusion dependence may be effective, even though it does not affect overall survival.
References
Bernard E, Tuechler H, Greenberg PL, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. N Eng J Med Evid. 2022;1(7):EVIDoa2200008.
Sauta E, Robin M, Bersanelli M, et al. Real-world validation of molecular international prognostic scoring system (IPSS-M) for myelodysplastic syndromes. Blood. 2022;140(Supplement 1):1121–4.
Aguirre LE, Al Ali N, Ball S, et al. Validation of the molecular international prognostic scoring system (IPSS-M) risk stratification model for myelodysplastic syndromes. Blood. 2022;140(Supplement 1):1125–7.
Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–19.
Arber DA, Orazi A, Hasserjian RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–28.
Ball S, Singh AM, Al Ali N, et al. A product of clash of titans or true reflection of disease biology? Validation of 2022 WHO and ICC classifications in a large dataset of patients with Myelodysplastic syndrome. Blood. 2022;140(Supplement 1):1118–20.
Haferlach C, Huber S, Mueller H, et al. MDS classification—do we still have to count blasts? Blood. 2022;140(Supplement 1):1130–1.
Platzbecker U, Santini V, Komrokji RS, et al. Characterization of patients with lower-risk myelodysplastic syndromes experiencing long-term responses with luspatercept in the MEDALIST study. Blood. 2022;140(Supplement 1):9808–10.
Santini V, Fenaux P, Zeidan AM, et al. Overall survival and progression-free survival of patients following luspatercept treatment in the MEDALIST trial. Blood. 2022;140(Supplement 1):4079–81.
Komrokji RS, Al Ali N, Ball S, et al. Luspatercept for treatment of lower risk myelodysplastic syndromes: real world data replicates medalist study results and confirms activity among hypomethylating agents and lenalidomide treated patients. Blood. 2022;140(Supplement 1):4039–41.
Mukherjee S, Brown-Bickerstaff C, McBride A, et al. Real-world outcomes of patients with lower-risk myelodysplastic syndromes (LR-MDS) treated with luspatercept: an evaluation of US clinical practice utilization and treatment patterns. Blood. 2022;140(Supplement 1):944–6.
Platzbecker U, Della Porta MG, Santini V, et al. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial. Lancet. 2023. https://doi.org/10.1016/S0140-6736(23)00874-7.
López Cadenas F, Lumbreras E, González T, et al. Evaluation of lenalidomide (LEN) vs placebo in non-transfusion dependent low risk del(5q) MDS patients. Final results of sintra-REV phase III international multicenter clinical trial. Blood. 2022;140(Supplement 1):1109–11.
Toribio Castelló SM, Lopez-Cadenas F, Preudhomme C, et al. Long-term evolution of somatic mutations in patients with del(5q) MDS early treated with lenalidomide in the sintra-rev clinical trial: safe and effecitive approach? Blood. 2022;140(Supplement 1):9740–3.
Sperling AS, Guerra VA, Kennedy JA, et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood. 2022;140(16):1753–63.
Platzbecker U, Komrokji RS, Fenaux P, et al. Imetelstat achieved prolonged, continuous transfusion independence (TI) in patients with heavily transfused non-del(5q) lower-risk myelodysplastic syndrome (LR-MDS) relapsed/refractory (R/R) to erythropoiesis stimulating agents (ESas) within the Imerge phase 2 study. Blood. 2022;140(Supplement 1):1106–8.
Platzbecker U, Santini V, Fenaux P, et al. Continuous tranfusion independence with Imetelstat in heavily transfused NON-DEL(5Q) lower-risk myelodysplastic syndromes relapsed/refractory to Erythropoiesis stimulating agents in Imerge phase 3 topic: MPN and MDS: targeting red cells and platelets. 2023; 165.
Zeidan AM, Ando K, Rauzy O, et al. Primary results of stimulus-MDS1: a randomized, double-blind, placebo-controlled phase II study of TIM‑3 inhibition with sabatolimab added to Hypomethylating agents (HMas) in adult patients with higher-risk Myelodysplastic syndromes (MDS). Blood. 2022;140(Supplement 1):2063–5.
Brunner AM, Esteve J, Porkka K, et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMas) in patients (pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute Myeloid leukemia (AML): final analysis from a phase Ib study. Blood. 2021;138(Supplement 1):244–244.
Garcia-Manero G, McCloskey J, Griffiths EA, et al. Pharmacokinetic exposure equivalence and preliminary efficacy and safety from a randomized cross over phase 3 study (ASCERTAIN study) of an oral Hypomethylating agent ASTX727 (cedazuridine/decitabine) compared to IV Decitabine. Blood. 2019;134(Supplement_1):846.
Bernard E, Nannya Y, Hasserjian RP, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26(10):1549–56.
Savona MR, McCloskey JK, Griffiths EA, et al. Prolonged survival in Bi-allelic TP53-mutated (TP53mut) MDS subjects treated with oral decitabine/cedazuridine in the ascertain trial (ASTX727-02). Blood. 2022;140(Supplement 1):2066–9.
Takahashi K, Patel K, Bueso-Ramos C, et al. Clinical implications of TP53 mutations in myelodysplastic syndromes treated with hypomethylating agents. Oncotarget. 2016;7(12):14172–87.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. Petzer and D. Wolf declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petzer, V., Wolf, D. FlASHback—personal highlights regarding myelodysplastic syndrome from the 2022 ASH meeting. memo 16, 152–155 (2023). https://doi.org/10.1007/s12254-023-00900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-023-00900-6