Summary
Colorectal cancer (CRC) is one of the most frequent malignancies. While adjuvant fluoropyrimidine-based chemotherapy has been established as standard of care for patients with stage III disease, its value and role are still uncertain for stage II disease. This review discusses the usefulness of adjuvant therapy in both stages and highlights the use of liquid biopsy via circulating tumor DNA (ctDNA) for the assessment of minimal residual disease which will shape the therapy decision for adjuvant treatment in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) is the second most common malignant tumor in women and the third in men in German-speaking countries. The mean age of onset is between 70–75 years. Individuals with genetic predisposition may develop the disease in early adulthood. With globally almost 2 million new cases and 1 million deaths worldwide in 2020, it can be considered a global burden [1]. The prognosis and choice of therapy of patients with CRC depends on the stage of the disease at initial diagnosis and other biological risk factors. For locally confined CRC in stages I–III, surgery is the cornerstone of treatment. In addition, in stage III and in subgroups of stage II adjuvant chemotherapy may be chosen [2].
Role of chemotherapy in stage II and III colorectal cancer
While adjuvant fluoropyrimidine-based chemotherapy has been established as standard of care for patients with stage III disease, its value and role are still uncertain for stage II disease. In 80% of patients, surgery alone can be a curative option; however, no clear benefit could be observed in trials of adjuvant therapy [3]. Particularly in borderline cases of stage II patients with high microsatellite instability (MSI-high or MSI-H) and concurrent high-risk features such as T4 disease, intestinal obstruction, fewer than 12 lymph nodes harvested, poorly differentiated histology, invasion (vascular, lymphatic, or perineural), or tumor perforation, adjuvant chemotherapy is subject of ongoing debate and controversy [4]. It has however been observed that some patients with these “high-risk features” might not necessarily have disease recurrence, whereas some patients with a cancer considered “low-risk” in fact do [5]. Thus, there is an urgent need for better biomarkers to predict the efficacy and necessity of chemotherapy and to predict recurrence risk after surgery for stage II colon cancer.
Following chemotherapy, it has been observed that approximately one third of patients drop out of therapy prematurely due to high toxicity and low quality of life. In 2020, the International Duration Evaluation of Adjuvant Therapy (IDEA) group advocated a shortening of adjuvant chemotherapy in patients with low-risk stage III colon cancer [6]. Their study examined the association between patient groups receiving either FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) administered for 3 versus 6 months in over 12,000 patients with stage III CRC. In the overall evaluation, 3 months of chemotherapy was not non-inferior to 6 months of postoperative treatment in terms of disease-free survival (DFS). In a subgroup analysis of patients with low-risk stage III cancer (T1–3 plus N1), the combination of capecitabine with oxaliplatin (CAPOX) for 3 months was in fact non-inferior to 6 months. In these patients the incidence of neurotoxicity, hand–foot syndrome, mucositis, nausea, fatigue, and diarrhea occurred significantly less frequently after 3 than after 6 cycles, as expected [6].
To provide more evidence on the prognostic impact of early therapy discontinuation and duration of adjuvant chemotherapy, the ACCENT/IDEA systematic review and meta-analysis presented their results at ASCO in 2022. Pooled data were obtained from 11 studies of 10,444 patients that were planned to receive 6 months of adjuvant fluoropyrimidine plus oxaliplatin (FOLFOX or CAPOX) and were analyzed for early discontinuation of all therapy or early discontinuation of only oxaliplatin therapy. Around 20.9% of patients discontinued all therapy regimes; 18.8% of patients discontinued oxaliplatin therapy with continuous 5‑fluorouracil (5-FU)/capecitabine administration. Patients who discontinued early were mainly female, aged ≥ 65 years, with poorer general health (ECOG PS ≥ 1), with body mass index (BMI) < 18.5, and patients on the CAPOX regimen (vs. FOLFOX) discontinued both therapy and oxaliplatin more frequently. For patients who only discontinued oxaliplatin therapy early, neither DFS nor overall survival (OS) was significantly different when up to 75% of cycles were received. There was however a significant difference in DFS when < 50% of oxaliplatin cycles were received (p < 0.001). According to these results, when neurotoxicity (grade 1–2) occurs, the discontinuation of oxaliplatin after 3 months of therapy may not affect the success of therapy and may therefore be a valid option. Simultaneously, these findings also undermine the importance of fluoropyrimidine-based therapy in the adjuvant treatment of localized CRC [7].
Role of liquid biopsy in detecting circulating tumor DNA
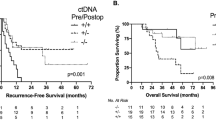
As mentioned, in current clinical practice the indication for adjuvant chemotherapy is still being debated and markers for recurrence prediction and DFS are being investigated. It has been shown that during necrosis or apoptosis of tumor cells, DNA segments are released into the bloodstream and can be detected as circulating tumor DNA (ctDNA) [8]. Several independent studies have demonstrated the prognostic significance of measuring ctDNA postoperatively [9]. Patients with postoperative ctDNA positivity are considered as having minimal residual disease (MRD) and thereby having clinically relevant and statistically significant shorter DFS than patients with postoperative ctDNA negativity [9]. Based on these important results, it was Taieb et al. who measured ctDNA from CRC patients included in the IDEA-FRANCE phase III trial to assess its prognostic and predictive value in the course of the adjuvant therapy [10]. It was observed that postoperative ctDNA positivity was associated with poor tumor differentiation, T4, tumor perforation and shorter 2‑year DFS with ctDNA being an independent prognostic marker. There was a significant difference in the 2‑year DFS in patients with ctDNA positivity with 64%, compared to 82% in patients with ctDNA negativity (hazard ratio [HR] 1.75 95% confidence interval [CI] 1.25–2.45; p = 0.001). Interestingly, ctDNA-positive patients treated with 6 months adjuvant therapy had a similar prognosis to ctDNA-negative patients given only 3 months of therapy [10].
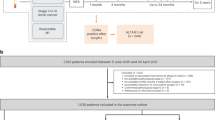
Taking these findings further, the DYNAMIC phase III trial investigated whether postoperative ctDNA can be used as a selection criterion for adjuvant therapy in patients diagnosed with stage II CRC. Choice of adjuvant therapy was based in one group on ctDNA positivity, in the other on clinical risk factors with 2‑year recurrence-free survival (RFS) being the primary endpoint. A total of 455 patients underwent 2:1 randomization. In the ctDNA patient group, 15% of the patients received adjuvant chemotherapy compared to 28% in the control group. The ctDNA-based treatment decision with 93.5% was non-inferior to the standard treatment with 92.4% in terms of 2‑year RFS. The 3‑year RFS was 86.4% in ctDNA-positive patients who received adjuvant chemotherapy compared to 92.5% in ctDNA-negative patients who did not receive therapy (HR 1.83; 95% CI 0.79–4.27). These promising results show that treatment decisions guided by ctDNA results compared to decisions based on standard clinicopathological features reduced the necessity for adjuvant chemotherapy without compromising recurrence-free survival [5].
It has been a concern that high levels of cell-free DNA (cfDNA) originating from normal tissue after surgery may prevent adequate detection of ctDNA. Thus, a recent study from Cohen et al. retrospectively analyzed real-world data from 16,347 patients to further understand how cfDNA levels and the timing of blood samples may impact minimal residual disease (MRD monitoring) [11]. Immediately after surgery, levels of cfDNA postoperatively were highest, gradually declined over the next weeks (p < 0.0001) and did not appear to significantly affect ctDNA detection. When concentrations were higher in the first 2 weeks, ctDNA was detected in approximately 18% of patients. When analyzing the data > 2 weeks postoperatively, ctDNA detection rates were more consistent. Moreover, ctDNA positivity in 2–8 weeks after surgery and > 6 months surveillance was associated with significantly worse recurrence-free survival compared with patients who were negative for ctDNA (p < 0.0001). Overall, these data could suggest that a standard testing window could start as early as 15 days postoperatively to not further delay adjuvant therapy, as well as guide clinical trial designs using ctDNA as an integral biomarker [11].
Currently, the CIRCULATE study is being conducted in 14 centers across Germany and Austria. In case of ctDNA positivity, patients are randomized to a standard therapy arm (5-FU-based therapy for 3–6 months), while patients with postoperative ctDNA negativity are followed up. These results will give further information on whether ctDNA-guided decision making is a suitable approach without compromising disease-free survival or overall survival and maybe even open the idea to a ctDNA-based follow-up regime.
Conclusion
For adequate decision making in the adjuvant management of CRC patients, various factors and variables including age, stage, gender, sidedness, molecular profile, minimal residual disease, type of adjuvant therapy, therapy duration, and patient’s wish have to be considered and integrated into clinical routine. This stratification might spare unnecessary toxicity to patients in the adjuvant setting by optimizing the prognosis. Future molecular profiling ideally assessed and monitored by liquid biopsy might even more personalize decision making in the adjuvant setting of CRC patients. Further research and clinical trials are needed to clarify relevant questions and to identify important clinical aspects.
References
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338.
Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–305.
Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54(1):5–16.
Verhoeff SR, van Erning FN, Lemmens VEPP, de Wilt JHW, Pruijt JFM. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139(1):187–93.
Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–72.
Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–88.
Gallois C, Shi Q, Meyers JP, Iveson T, Alberts SR, de Gramont A, et al. Prognostic impact of early treatment and oxaliplatin discontinuation in patients with stage III colon cancer: an ACCENT/IDEA pooled analysis of 11 Adjuvant trials. J Clin Oncol. 2023;41(4):803–15.
Stejskal P, Goodarzi H, Srovnal J, Hajdúch M, van ’t Veer LJ, Magbanua MJM. Circulating tumor nucleic acids: biology, release mechanisms, and clinical relevance. Mol Cancer. 2023;22(1):15.
Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
Taieb J, Valerie TA, Vernerey D, Bourreau C, Bennouna J, Faroux R, et al. LBA30_PRAnalysis of circulating tumour DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: prognostic and predictive value for adjuvant treatment duration. Ann Oncol. 2019; https://doi.org/10.1093/annonc/mdz394.019.
Cohen SA, Kasi PM, Aushev VN, Hanna DL, Botta GP, Sharif S, et al. Kinetics of postoperative circulating cell-free DNA and impact on minimal residual disease detection rates in patients with resected stage I–III colorectal cancer. J Clin Oncol. 2023;41(4):5.
Funding
Open access funding provided by Karl Landsteiner Privatuniversität für Gesundheitswissenschaften
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Schmalfuss and H. Taghizadeh declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmalfuss, T., Taghizadeh, H. Lessons learned in adjuvant colorectal cancer. memo 16, 113–115 (2023). https://doi.org/10.1007/s12254-023-00882-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-023-00882-5