Summary
Inhibitors of the cyclin-dependent kinases 4/6 (CDK4/6i) have been practice-changing and are now considered the standard of care in combination with endocrine therapy for the first- or second-line treatment in advanced hormone-receptor-positive, human epidermal growth factor receptor 2‑negative breast cancer. Recently, CDK4/6i have also emerged as an appealing targeted cancer therapy in early breast cancer, however results of large clinical trials are controversial. This position paper summarizes the evidence, and provides guidance for clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inhibitors of the cyclin-dependent kinases 4/6 (CDK4/6i) have been practice-changing and are now considered the standard of care in combination with endocrine therapy (ET) for the first- or second-line treatment in advanced hormone-receptor-positive (HR+), human epidermal growth factor receptor 2‑negative (HER2−) breast cancer (BC) [1,2,3,4,5,6,7]. Recently, CDK4/6i have also emerged as an appealing targeted cancer therapy in early breast cancer [8].
Therefore, this position paper aims to provide up-to-date information on biomarker analyses to better select patients who may derive the greatest benefit from CDK4/6i treatment as well as appraise the role of CDK4/6i in early breast cancer (EBC), and to highlight ongoing research.
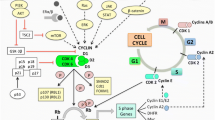
Breast cancer patient-derived cell lines have demonstrated greater dependence on CDK4 versus CDK6 since CDK6-dependent lines are mostly hematopoietic in origin [9]. Additionally, CDK4 mRNA is expressed at significantly higher levels than CDK6 in estrogen-receptor-positive (ER+) breast cancer samples [10]. Consistent with prior biochemical studies and cell proliferation assays, knockout models indicate that both ribociclib and abemaciclib more potently inhibit CDK4 than CDK6, whereas palbociclib has similar activity against both targets in cells [11]. In more detail, the half maximal inhibitory concentrations (IC50) in preclinical drug-exposure experiments showed a CDK4 to CDK6 ratio of 1:1.5 for palbociclib (9–11 nM and 15 nM, respectively), 1:5 for abemaciclib (2 nM and 9.9 nM, respectively) and 1:4 for ribociclib (10 nM and 39 nM, respectively; Table 1). The pharmacokinetic profiles of the three CDK4/6i are also depicted in Table 1. After rapid absorption and distribution, the maximum concentration (Cmax) is achieved within 6–12 h for palbociclib, 8 h for abemaciclib and 1–4 h for ribociclib [12]. The elimination half-life (T1/2), especially important to predict either the onset of action or recovery upon treatment discontinuation due to toxicities, varies between 24–34 h for palbociclib, 17–38 h for abemaciclib, and 30–55 h for ribociclib [12]. Since all three CDK4/6i are predominantly metabolized by CYP3A4 in the liver, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) advise to avoid concomitant use of strong CYP3A4 inhibitors and CDK4/6i [13,14,15]. Interestingly, abemaciclib seems to be more lipophilic possibly enabling it to penetrate breast tissue and the blood–brain barrier more efficiently compared to the other two CDK4/6i [12]. Thus, understanding the importance of CDK4 relative to CDK6 as a driver of tumor cell proliferation and possible implications for increased CDK4 target engagement is an emerging topic of discussion that may have important implications.
Prognostic value of intrinsic subtypes for CDK4/6 inhibition therapy
With the development of microarrays, gene expression profiling has been used to identify patients with sufficiently good, intermediate, or poor prognosis. Based on pioneer studies by Sørlie et al., tumors were classified into five intrinsic subtypes with distinct clinical outcomes, thereby reflecting fundamental differences at the molecular level, i.e., luminal A, luminal B, HER2-enriched (HER2E), basal, and normal-like [18,19,20].
Among these subtypes, data from patients who provided metastatic tumor samples in PALOMA‑3 indicate that palbociclib is equally efficient in luminal A and B tumors (hazard ratio [HR] for progression-free survival [PFS], 0.23 [95% CI 0.11–0.47] and 0.26 [95% CI 0.12–0.56], respectively)[21], whereas in the MONALEESA studies, ribociclib was even more efficient in the HER2E subtype (HR for PFS, 0.39 [95% CI 0.25–0.60]), accounting for 12.7% of the study population, compared to luminal A and B (HR for PFS, 0.63 [95% CI 0.49–0.83] and 0.52 [95% CI 0.25–0.60], respectively) but not efficient in basal-like subtypes (HR for PFS, 1.15 [95% CI 0.46–2.83]) [22]. This was reflected in the overall response rate [22]. Collectively, the HER2E subtype showed the highest levels of ERBB2 mRNA and protein, appeared to be the subtype with the highest activation of the EGFR-HER2 signaling pathway, and had worse response compared to luminal subtypes [23,24,25].
Biomarkers of response and/or resistance to CDK4/6 inhibitors
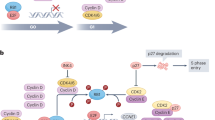
Understanding the endocrine and/or CDK4/6 resistance mechanisms, identifying reliable predictive biomarkers and novel treatment combinations to overcome resistance are urgently needed since approximately 20% of patients treated with combined CDK4/6i and ET exhibit primary resistance, while acquired (secondary) resistance eventually develops and limits efficacy in almost all patients [26]. Although still poorly understood, some of the suggested resistance mechanisms to combined CDK4/6i plus ET are similar to those following progression on ET monotherapy (e.g. PIK3CA and ESR1 mutations or upregulation of CDK6) [5, 27,28,29]. In general, they can be categorized into two broad groups: cell-cycle-specific and noncell-cycle-specific mechanisms [30, 31].
Among the cell-cycle-related biomarkers, loss of RB was associated with worse prognosis in patients treated with palbociclib plus ET [32]. In patients with low cyclin E1 (CCNE1) expression, the addition of palbociclib to fulvestrant demonstrated the highest PFS benefit compared to high CCNE1 expression or placebo plus fulvestrant [33]. In cell culture models, amplification of CDK4 or CDK6 expression was linked to reduced response of the CDK4/6 target, phospho-RB, to CDK4/6i [34].
Moreover, a gene signature of E2F activity was shown to discriminate between palbociclib-sensitive and -resistant cell lines [35], and high expression of E2F target genes was associated with lack of PFS improvement upon palbociclib therapy [33]. Activity of the serum thymidine kinase (TKa) that is located downstream of the CDK4/6 pathway seems to be a dynamic marker of outcomes in patients treated with palbociclib. Thus, high pretreatment TKa and its incomplete suppression during treatment may identify patients with primary resistance and worse prognosis [36, 37].
Regarding other noncell-cycle-dependent mechanisms, the expression of fibroblast growth factor receptor 1 (FGFR1) was associated with resistance to fulvestrant ± ribociclib or palbociclib in cell culture models [38]. Additionally, in ER+ breast cancer treated with CDK4/6i, FGFR alterations correlated with poor outcome, which was reflected by shorter PFS in patients with cancers with high tumor FGFR1 mRNA expression treated with letrozole/ribociclib compared to patients with tumors with low tumor FGFR1 mRNA expression [38]. In this regard, the presence of a deleterious FAT1 mutation in tumors of patients treated with a CDK4/6i was also associated with decreased median PFS [39].
Recently, serial circulating tumor DNA (ctDNA) analyses brought additional insights regarding the biology of CDK4/6i response by detecting the acquisition of new alterations in ERBB2, PIK3CA, AKT1, MYC, CCND1, and ESR1. Although these alterations might have emerged due to prolonged exposure to CDK4/6i plus ET, patients carrying ESR1 mutations prior to treatment were unlikely to benefit from the combination therapy [40]. Although very few patients were included in this study, ctDNA analysis detected tumor-derived mutations indicative of disease progression well in advance of radiologic assessments [40].
Transcriptomics, proteomics and multigene assays (e.g. MammaPrint, Oncotype DX, PAM50) are powerful tools yielding molecular tumor profiles; however, the majority of these data were derived from retrospective analyses from individual clinical trials, and it is still unclear if and how they can inform clinical decision-making in the future. Recently, a high throughput genomic profile was identified that suggests palbociclib activity in two early breast cancer trials, PALLAS and PENELOPE‑B [41]. Nevertheless, prospective clinical trials evaluating these new tools and their candidate patterns/profiles are urgently needed, since to date, no biomarker beyond HER2 and HR status has been firmly validated or convincingly demonstrated clinical utility in EBC [42].
CDK4/6 inhibitors as neoadjuvant therapy for early BC
The combination of ET and CDK4/6i has benefited patients with ER+/HER2− advanced breast cancer; however, its effects in the neoadjuvant setting for EBC are unclear.
Since 2016, many studies have explored the efficacy of CDK4/6i plus neoadjuvant ET, either versus ET alone or versus standard chemotherapy (CT) in patients with HR+/HER2− early-stage disease. The efficacy was estimated either by measuring Ki67, complete cell cycle arrest (CCCA; defined by a Ki67 proliferation index ≤ 2.7%), preoperative endocrine prognostic index (PEPI), and/or response rates [43,44,45,46,47,48,49,50]. Study data for neoadjuvant CDK4/6i therapy are summarized and discussed in the following paragraphs.
In the phase II MonaLEEsa‑1 window of opportunity study, all of the enrolled patients who received ET with or without ribociclib prior to surgery experienced a decreased proliferation rate, although they did not show a definitive advantage for the combination arm [51].
The phase II neoMONARCH trial assessed the biological effects of abemaciclib with or without ET compared to ET alone. A greater reduction in median Ki67 levels after 2 weeks was observed with abemaciclib alone (−91%) or with the combination of abemaciclib with anastrozole (−93%) compared with anastrozole alone (−63%). Likewise, CCCA was more commonly observed in the abemaciclib containing arms than with anastrozole alone. Interestingly, immune pathway gene sets were enriched in tumors treated with abemaciclib + ET, definitely warranting further investigation [49].
DxCARTES, a multicenter, open-label, noncomparative, phase 2 trial assessed the biological and clinical activity of palbociclib + ET as neoadjuvant treatment in HR+/HER2− EBC with an Oncotype DX recurrent score (RS) ≥ 18 (cohort A, RS 18–25; cohort B, RS 26–100, respectively). Strikingly, the coprimary endpoint was met in cohort B only, with > 50% achieving an RS ≤ 25 or a pathological complete response (pCR) at the time of surgery. Since there was an RS increase upon therapy in one third of tumor samples in cohort A compared to baseline, the question arose whether RS is a dynamic biomarker, rather than a static one [52].
In PALLET, a phase II randomized trial, adding palbociclib to ET did not affect the clinical response but markedly enhanced the suppression of malignant cell proliferation as assessed by Ki67 changes (log-fold as well as percentage). Also, compared with ET alone, it resulted in increased apoptosis as measured by the reduction of c‑PARP levels. Moreover, there was a significant increase in patients achieving CCCA in their tumors after 14 weeks of combination therapy compared with ET alone (90% vs 59%). No differences, however, were found between palbociclib + ET and ET alone in pCR rates or breast conservation rates [46].
Another important study, the single-arm phase II neoadjuvant NeoPalAna trial, not only demonstrated the potent antiproliferative effect of palbociclib when the response to ET alone was incomplete but also shed light on proliferation after palbociclib withdrawal. This was enabled by the study design, with a median washout period of 29 days for palbociclib before surgery, whereas ET was continued until surgery. Interestingly, Ki67 was significantly higher at the time of surgery upon palbociclib washout (after cycle 4) but not significantly different from baseline levels, and this rebound Ki67 effect at surgery was suppressed when palbociclib (cycle 5) was administered before surgery. This finding indicates that the antiproliferative effect of palbociclib is reversible despite 4 months of therapy. Therefore, the inhibition of proliferation by palbociclib + ET may be biologically distinct from the effect achieved with ET alone and this raises the question whether the PEPI score may be an appropriate endpoint for neoadjuvant CDKi trials [45].
A similar design was used in the FELINE trial comparing ET with or without ribociclib, which was either given continuously or intermittently. Patients with Ki67 > 10% after 2 weeks were removed from the trial as this population is expected to harbor an increased recurrence risk. The addition of ribociclib to ET did not result in a higher percentage of women with a PEPI score of 0. Although there was a numerical advantage of the combination in terms of clinical response, proliferation was significantly increased between day 14 of cycle 1 and surgery in the CDKi arm compared to ET alone, which indicates a possible escape mechanism [48].
Another approach compared CDK4/6i + ET with optimal chemotherapy. These studies were NeoPAL, a randomized, parallel, noncomparative phase II study investigating the potential role of palbociclib, and CORALLEEN, a parallel-arm, multicenter, randomized, open-label, phase 2 trial assessing ribociclib. Both studies showed that the pCR rate and clinical response rate were similar in both groups. A numerically higher proportion of patients in the chemotherapy arm compared to CDK4/6i + ET achieved RCB 0–I tumor responses according to local assessment [47, 50]. Moreover, with a median follow-up of 40 months in NeoPAL, there was no difference between the two study arms in terms of progression-free survival and invasive disease-free survival (secondary trial endpoints), suggesting that a neoadjuvant CDK4/6i + ET strategy may allow omission of chemotherapy in some patients while still enabling favorable long-term outcomes. However, larger confirmatory studies are definitely needed [53]. Interestingly, CORALLEEN showed conversion of the intrinsic subtype from luminal A to luminal B in both cohorts consistent with the primary endpoint, which was the proportion of patients with PAM 50 low-risk-of-relapse disease [50]. Since a small proportion converted to a HER2E subtype, this might again highlight that the subtype represents a dynamic marker influenced by treatment.
Ongoing studies including CARABELA (NCT04293393) will give insights into the efficacy of standard chemotherapy vs ET + abemaciclib as neoadjuvant therapy, and the PROMETEO II study (NCT04130152) explores whether ET + palbociclib before surgery offers advantages in patients with clinical residual disease after completing neoadjuvant chemotherapy.
Overall, studies consistently showed reduced proliferation upon neoadjuvant CDK4/6i plus ET therapy that was more pronounced than with ET alone but rebound effects were observed. The clinical activity of CDK4/6i plus ET was similar compared to chemotherapy, although these results need to be further investigated in larger phase III trials with long-term follow-up.
CDK4/6 inhibitors in the adjuvant setting of early BC
Four major trials (Table 2) have explored the role of CDK4/6i in the adjuvant therapy of EBC. While three of them, PENELOPE‑B [54] and PALLAS [55] assessing palbociclib and monarchE [8] investigating abemaciclib already yielded results from interim or final analyses, the results of NATALEE [56] that is testing ribociclib are expected for 2023. PALLAS, monarchE, and NATALEE include translational research programs analyzing ctDNA and tissue samples. At the SABCS 2022, Loibl et al. presented results from a prospectively defined retrospective analysis of a subset of intermediate/high-risk patients with EBC selected from PALLAS where the composite predictive biomarker (luminal A with ERBB2-high and/or luminal A ER+/progesterone receptor negative [PR−]), defined from PENELOPE‑B, was validated [41]. The HTG-AIMS intrinsic molecular subtype distribution was similar between PENELOPE‑B (luminal A, 73.2%) and PALLAS (luminal A, 72.7%) HTG sets. Interestingly, the biomarker-positive subgroup demonstrated a significant improvement in invasive disease-free survival (iDFS) with addition of palbociclib to ET in the PENELOPE‑B trial (HR, 0.63 [95% CI 0.42–0.95], p = 0.025) which was absent in the biomarker-negative subgroup (HR, 1.11 [95% CI 0.79–1.56], p = 0.56). The independent validation of the biomarker with tumor samples from the PALLAS HTG validation set confirmed the significant benefit from palbociclib + ET (HR, 0.55 [95% CI 0.34–0.90], p = 0.017) in the biomarker-positive compared to the biomarker-negative subgroup (HR, 1.37 [95% CI 0.99–1.88], p = 0.058), respectively. Of note, the significant treatment effects remained after multivariable analyses based on the trial stratification factors to adjust for potential baseline confounders [41]. Overall, these data further support the use of the composite predictive biomarker (luminal A with ERBB2-high and/or luminal A ER+/PR−) for patient stratification in future adjuvant clinical trials for treatment of HR+/HER2− EBC; however, the full publication and critical discussion of these results will have to be awaited.
Neither in the PALLAS study (pre- and postmenopausal women or men with stage II or stage III EBC) nor in the PENELOPE‑B trial (women with HR+/HER2− EBC considered at high risk of relapse after neoadjuvant CT) did palbociclib + ET statistically improve invasive disease-free survival (iDFS) compared to ET alone [54, 55]. However, in monarchE (pre- and postmenopausal women or men with high-risk early invasive breast cancer), abemaciclib demonstrated a 33.6% reduction in the risk of developing an iDFS event with an increase in absolute benefit in iDFS 4‑year rates of 6.5% compared to 2‑ and 3‑year iDFS rates of 2.8% and 4.8%, respectively [8, 61, 62]. Moreover, the 2‑,3-, and 4‑year distant relapse-free survival (dRFS) differed by 2.5%, 4.1%, and 5.9%, respectively, with a 34.1% reduction of relative risk of invasive disease [61, 62]. While OS data remain immature at 4 years, fewer deaths were observed with abemaciclib plus ET compared to ET alone [62].
Interestingly, treatment with abemaciclib + ET halved bone metastases and reduced liver metastases by one-third, whereas there was no effect on lung metastases [61].
Although the reasons for the differences in outcomes between these studies are unclear, several reasons have been postulated, including differences in patient selection, drug exposure, the drug itself, or the treatment schedule [58]. While the baseline characteristics of PALLAS and monarchE differ due to recruitment of more patients with higher-stage disease in monarchE (48.6% stage III in PALLAS vs 74.1% in monarchE), the overall proportion of patients with stage IIB or III did not differ (82.2% in PALLAS vs 88.0% in monarchE) [63]. Thus, even if the analysis of PALLAS focused on similarly higher-risk subgroups (IIB or III), no benefit was observed with palbociclib treatment [58]. Moreover, a linear and spline trend analysis of the PALLAS data conducted to analyze the interaction of composite risk and treatment (using the hazard ratio) asserted that neither lower- nor higher-risk patients benefit from adjuvant palbociclib [55].
Furthermore, there was a difference in treatment duration between the post-neoadjuvant PENELOPE‑B trial, where patients with residual disease were treated after neoadjuvant treatment for 1 year, and the PALLAS and monarchE trials, where patients were exposed to CDK4/6i for 2 years (Table 2).
Additionally, there was a difference in discontinuation rates before completing the 1‑ or 2‑year treatment period due to other reasons than recurrence between the trials. In the monarchE trial, 25.8% stopped abemaciclib (including 18.5% due to adverse events) before the planned 2‑year duration and an estimation using the Kaplan–Meier method revealed an estimated discontinuation rate at 6 months of approximately 14% and at 1 year of approximately 19.4%, respectively [59, 60]. In the PALLAS trial, 42.2% stopped palbociclib (including 27.2% due to adverse events) before the planned 2‑year duration and a post hoc analysis revealed a 6-month and 1‑year estimated cumulative rate of discontinuation of 17.9% and 30.0%, respectively [57]. However, these differences in study outcomes due to adherence are not supported by the results of PENELOPE‑B that demonstrated a discontinuation rate of 19.6% (including 5.3% due to adverse events) before completing 1 year of palbociclib treatment [54].
In terms of pharmacologic differences, abemaciclib was shown to have greater inhibitory activity on CDK4 over CDK6, as well as activity against CDK2 and CDK9 [16, 17]. However, there has been no head-to-head comparison between palbociclib and abemaciclib, and efficacy data from trials in the advanced setting were comparable in terms of their primary endpoint PFS [64, 65]. Of note, while the robust PFS benefit in MONARCH 3 led to global regulatory approval, overall survival (OS) was immature with 29.5% events observed across both arms at that time; final OS data are expected for 2023. At ESMO 2022, Goetz et al. presented the second interim analysis OS data of the intended to treat population, although still not statistically significant, the OS data trended favorably for the nonsteroidal aromatase inhibitor (NSAI) plus abemaciclib versus NSAI plus placebo (HR for OS, 0.754 [95% CI 0.584–0.974]) with an observed difference in median OS of 12.6 months [66]. However, in postmenopausal women with ER+/HER2− advanced BC (ABC), first-line palbociclib plus letrozole met its primary endpoint of improving PFS versus placebo plus letrozole, but not the secondary endpoint of OS (HR for OS, 0.956 [95% CI 0.777–1.177]) with an observed difference in median OS of 2.7 months [67]. For completeness, the final overall analysis of MONALEESA‑2, after a median follow-up of 6.6 years, showed not only significantly improved PFS but also improved overall survival with ribociclib plus letrozole versus letrozole alone as first-line treatment in postmenopausal patients with HR+/HER2− ABC (63.9 vs 51.4 months; hazard ratio [HR] = 0.76; 95% CI 0.63–0.93; two-sided p = 0.008) [68].
Probably, the pivotal difference in complete senescence and irreversible effects through apoptosis upon CDK4/6i treatment rests on the treatment schedule, with abemaciclib being given continuously whereas palbociclib and ribociclib are given in a 3-week-on, 1‑week off schedule. Targeting of proliferating cells in early breast cancer rather than inhibition of awakening of dormant cells could explain the differences in outcomes. At this point, however, the tie-breaker results of NATALEE with 400 mg ribociclib + ET (d1–d21 q28 for 3 years) are awaited. Until then, this remains scientific speculation [56].
Other approaches include the ADAPTcycle (NCT04055493) trial evaluating ribociclib + ET compared to standard-of-care chemotherapy followed by adjuvant ET, and the ADAPTlate (NCT04565054) study where patients with high risk of relapse according to Oncotype® (Exact Sciences, Madison, WI, USA) who received standard of care chemotherapy are randomized to either abemaciclib + ET or ET alone (Table 3; [69, 70]).
Discussion and conclusion
The blockade of the cell cycle with CDK4/6 inhibitors represents a fundamental new treatment approach to improve ET. While the role of CDK4/6i is well established in the advanced setting, emerging topics of discussion include the role of CDK4/6i in early breast cancer, the importance of CDK4 relative to CDK6 in the etiology of breast cancer, and possible implications for increased CDK4 target engagement.
Although a lot of data have already been gathered, an important question arising from the study data is whether estimation of proliferation by measuring Ki67, CCCA (defined by a Ki67 proliferation index ≤ 2.7%) and PEPI is adequate and meaningful in patients treated with CDK4/6i since these biomarkers were validated for either ET (PEPI score) or chemotherapy (Ki67). During the PALLAS trial, a huge biomaterial collection of specimens has been acquired for translational research (ongoing and planned), enabling future validation of biomarker assays and predefined signatures due to RNA sequencing, high-throughput genomic sequencing, or circulating tumor DNA analysis. With the help of such samples, a subset of EBC patients (luminal A with ERBB2-high and/or luminal A ER+/PR−) who derive benefit from the addition of palbociclib to ET has recently been identified [41].
While data from the adjuvant PlanB (Oncotype DX®/Recurrence Score®) and the MINDACT (MammaPrint®) trial may be helpful in guiding/refining CT decisions in HR+/HER2− EBC based on genomic signature and clinicopathological factors, particularly in pN0-1 patients otherwise considered as intermediate-to-high risk, additional targeted therapies, especially CDK4/6i, need to be evaluated in patients with high genomic risk who have significant residual risk despite adjuvant CT [71, 72]. Although in PlanB, the highly reproducible Oncotype DX®/Recurrence Score® outperformed immunohistochemistry markers as a prognostic factor in EBC [72], the question remains if there are more specific biomarkers able to differentiate between a quiescent and a senescent state that would not only be better suited but, more importantly, be easily implementable in clinical routine.
Other important information that will likely emerge with long-term follow-up data as well as data from NATALEE include patient-reported quality-of-life outcomes, the optimal definition of a “high-risk” patient population that might benefit the most, the impact on late recurrences, and of course the optimal duration of CDK4/6 inhibitor treatment.
Lastly, another main challenge will be to elucidate the full range of molecular resistance mechanisms given that a substantial fraction of tumors presents with pre-existing, intrinsic resistance to CDK4/6i and that some patients initially respond to CDK4/6i treatment, thereafter, develop resistance and eventually die from the disease.
Overall, the expert panel aligns with the ASCO Guidelines for adjuvant abemaciclib plus ET in the treatment of high-risk early breast cancer, specifically highlighting the benefits of abemaciclib across patients with varying Ki67 scores while being reserved about a potential role of CDK4/6i in the neoadjuvant setting given the excellent pCR rates and survival data of the WSG-ADAPT study.
References
Finn RS, Martin M, Rugo HS, Jones S, Im S‑A, Gelmon K, et al. Palbociclib and Letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36.
Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor—Positive advanced breast cancer. N Engl J Med. 2015;373:209–19.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35.
Hortobagyi GN, Stemmer SM, Burris HA, Yap Y‑S, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S‑A, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39.
Johnston S, Martin M, di Leo A, Im S‑A, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
Slamon DJ, Neven P, Chia S, Fasching PA, de Laurentiis M, Im S‑A, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone receptor—Positive, human epidermal growth factor receptor 2—Negative advanced breast cancer: MONALEESA‑3. J Clin Oncol. 2018;36:2465–72.
Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–81.
Kim S, Tiedt R, Loo A, Horn T, Delach S, Kovats S, et al. The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget. 2018;9:35226–40.
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Delach S, Caponigro G. Abstract PS19-10: Preclinical head-to-head comparison of CDK4/6 inhibitor activity toward CDK4 vs CDK6. Cancer Res. 2021;81:PS19-10.
Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in breast cancer with Palbociclib, Ribociclib, and Abemaciclib: similarities and differences. Drugs. 2021;81:317–31.
EMA Administration. Summary of product characteristics abemaciclib.. https://www.ema.europa.eu/en/documents/product-information/verzenios-epar-product-information_en.pdf. Accessed: January 2023.
EMA Administration. Summary of product characteristics palbociclib.. https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf. Accessed: January 2023.
EMA Administration. Summary of product characteristics ribociclib.. https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf. Accessed: January 2023.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46.
Chen P, v. Lee N, Hu W, Xu M, Ferre RA, Lam H, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15:2273–81.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Finn RS, Cristofanilli M, Ettl J, Gelmon KA, Colleoni M, Giorgetti C, et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in patients with bone-only disease: a joint analysis of PALOMA‑2 and PALOMA‑3 clinical trials. Breast Cancer Res Treat. 2020;184:23–35.
Prat A, Chaudhury A, Solovieff N, Paré L, Martinez D, Chic N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–67.
Creighton C. The molecular profile of luminal B breast cancer. Biologics. 2012; https://doi.org/10.2147/btt.s29923.
Cheang MCU, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015;20:474–82.
Eroles P, Bosch A, Alejandro Pérez-Fidalgo J, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707.
Al-Qasem AJ, Alves CL, Ehmsen S, Tuttolomondo M, Terp MG, Johansen LE, et al. Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. NPJ Precis Oncol. 2022;6:68.
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to Palbociclib plus Fulvestrant in the PALOMA‑3 trial. Cancer Discov. 2018;8:1390–403.
Gyanchandani R, Kota KJ, Jonnalagadda AR, Minteer T, Knapick BA, Oesterreich S, et al. Detection of ESR1 mutations in circulating cell-free DNA from patients with metastatic breast cancer treated with palbociclib and letrozole. Oncotarget. 2017;8:66901–11.
del Re M, Crucitta S, Lorenzini G, de Angelis C, Diodati L, Cavallero D, et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer patients. Pharmacol Res. 2021;163:105241.
Pandey K, An H, Kim SK, Lee SA, Kim S, Lim SM, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer. 2019;145:1179–88.
Roberto M, Astone A, Botticelli A, Carbognin L, Cassano A, D’Auria G, et al. CDK4/6 inhibitor treatments in patients with hormone receptor positive, her2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel). 2021;13:332.
Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat Commun. 2017;8:15916.
Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 expression and Palbociclib efficacy in previously treated hormone receptor—Positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–78.
Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–64.
Malorni L, Piazza S, Ciani Y, Guarducci C, Bonechi M, Biagioni C, et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget. 2016;7:68012–22.
Malorni L, Tyekucheva S, Hilbers FS, Ignatiadis M, Neven P, Colleoni M, et al. Serum thymidine kinase activity in patients with hormone receptor-positive and HER2-negative metastatic breast cancer treated with palbociclib and fulvestrant. Eur J Cancer. 2022;164:39–51.
McCartney A, Bonechi M, de Luca F, Biagioni C, Curigliano G, Moretti E, et al. Plasma thymidine Kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TRend trial. Clin Cancer Res. 2020;26:2131–9.
Formisano L, Lu Y, Servetto A, Hanker AB, Jansen VM, Bauer JA, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10:1373.
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34:893–905.e8.
Chin YM, Shibayama T, Chan HT, Otaki M, Hara F, Kobayashi T, et al. Serial circulating tumor DNA monitoring of CDK4/6 inhibitors response in metastatic breast cancer. Cancer Sci. 2022;113:1808–20.
Loibl S, Denkert C, Yuan L, Knudsen ES, DeMichele A, Zhang Z, et al. Development and validation of a composite biomarker predictive of palbociclib + endocrine treatment benefit in early breast cancer: PENELOPE‑B and PALLAS trials. In: Presented at SABCS 2022. December 6–10. 2022. Abstract PD17-05.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2017;2017(28):1700–12.
Hurvitz SA, Park YH, Bardia A, Quiroga V, López-Valverde V, Steinseifer J, et al. LBA14 Neoadjuvant giredestrant (GDC-9545) + palbociclib (palbo) vs anastrozole (A) + palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2− eBC): Interim analysis of the randomised, open-label, phase II coopERA BC study. Ann Oncol. 2021;32:S1285–S6.
Chow LWC, Morita S, Chow CYC, Ng W‑K, Toi M. Neoadjuvant palbociclib on ER+ breast cancer (N007): clinical response and EndoPredict’s value. Endocr Relat Cancer. 2018;25:123–30.
Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor—positive breast cancer. Clin Cancer Res. 2017;23:4055–65.
Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor—positive early breast cancer: PALLET trial. J Clin Oncol. 2019;37:178–89.
Cottu P, D’Hondt V, Dureau S, Lerebours F, Desmoulins I, Heudel P‑E, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29:2334–40.
Khan QJ, O’Dea A, Bardia A, Kalinsky K, Wisinski KB, O’Regan R, et al. Letrozole + ribociclib versus letrozole + placebo as neoadjuvant therapy for ER+ breast cancer (FELINE trial). J Clin Oncol. 2020;38:505–505.
Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2− breast cancer. Clin Cancer Res. 2020;26:566–80.
Prat A, Saura C, Pascual T, Hernando C, Muñoz M, Paré L, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33–43.
Curigliano G, Gómez Pardo P, Meric-Bernstam F, Conte P, Lolkema MP, Beck JT, et al. Ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast. 2016;28:191–8.
Llombart-Cussac A, Guerrero-Zotano Á, Ruiz M, Bermejo B, Gil-Gil M, de la Haba J, et al. Abstract P5-16-12: Neoadjuvant letrozole plus palbociclib in patients (pts) with hormone receptor (HR)-positive/HER2-negative early breast cancer (EBC) with baseline Ki67 ≥20 % and an Oncotype DX breast recurrence score® result (RS) ≥18: dxCARTES. Cancer Res. 2022;82:P5-16-12.
Delaloge S, Dureau S, D’Hondt V, Desmoulins I, Heudel P‑E, Duhoux FP, et al. Survival outcomes after neoadjuvant letrozole and palbociclib versus third generation chemotherapy for patients with high-risk oestrogen receptor-positive HER2-negative breast cancer. Eur J Cancer. 2022;166:300–8.
Loibl S, Marmé F, Martin M, Untch M, Bonnefoi H, Kim S‑B, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the Penelope‑B trial. J Clin Oncol. 2021;39:1518–30.
Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22:212–22.
Slamon DJ, Fasching PA, Patel R, Verma S, Hurvitz SA, Chia SKL, et al. NATALEE: Phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor—positive (HR+), human epidermal growth factor receptor 2—negative (HER2−) early breast cancer (EBC). J Clin Oncol. 2019;37:TPS597.
Mayer EL, Fesl C, Hlauschek D, Garcia-Estevez L, Burstein HJ, Zdenkowski N, et al. Treatment exposure and discontinuation in the PALbociclib coLlaborative adjuvant study of palbociclib with adjuvant endocrine therapy for hormone receptor—positive/human epidermal growth factor receptor 2—negative early breast cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03). J Clin Oncol. 2022;40:449–58.
Gnant M, Dueck AC, Frantal S, Martin M, Burstein HJ, Greil R, et al. Adjuvant palbociclib for early breast cancer: the PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol. 2022;40:282–93.
Rugo HS, O’Shaughnessy J, Boyle F, Toi M, Broom R, Blancas I, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol. 2022;33:616–27.
Tolaney SM, Johnston SRD, Wei R, Andre VA, Shahir A, Harbeck N, et al. Adjuvant abemaciclib for high-risk early breast cancer (EBC): Factors increasing the rate of treatment discontinuations in monarchE. J Clin Oncol. 2022;40:527–527.
Toi M, Boyle F, Im Y‑H, Reinisch M, Molthrop D, Jiang Z, et al. 59MO Adjuvant abemaciclib combined with endocrine therapy (ET): Efficacy results in monarchE cohort 1. Ann Oncol. 2022;33:S149.
Johnston S, Toi M, O’Shaughnessy J. Abemaciclib plus endocrine therapy for HR+, HER2−, node-positive, high-risk early breast cancer: results from a pre-planned monarchE overall survival interim analysis, including 4‑year efficacy outcomes. In: Presented at SABCS 2022. December 6–10. 2022. Abstract GS1-09.
Agostinetto E, Vian L, Caparica R, Bruzzone M, Ceppi M, Lambertini M, et al. CDK4/6 inhibitors as adjuvant treatment for hormone receptor-positive, HER2-negative early breast cancer: a systematic review and meta-analysis. ESMOo Open. 2021;6:100091.
di Cosimo S, Porcu L, Cardoso F. CDK 4/6 inhibitors mired in uncertainty in HR positive and HER2 negative early breast cancer. Breast. 2021;55:75–8.
Agostinetto E, Caparica R, de Azambuja E. CDK4/6 inhibition in HR-positive early breast cancer: are we putting all eggs in one basket? ESMO Open. 2020;5:e1132.
Goetz M. MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2− advanced breast cancer (ABC). Ann Oncol. 2022;33(suppl_7; Abstr LBA15):S808–S69.
Finn RS, Rugo HS, Dieras VC, Harbeck N, Im S‑A, Gelmon KA, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor—positive/human epidermal growth factor receptor 2—negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA‑2. J Clin Oncol. 2022;40:LBA1003–LBA1003.
Hortobagyi GN, Stemmer SM, Burris HA, Yap Y‑S, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942–50.
Gluz O, Scheffen I, Degenhardt T, Marschner NW, Christgen M, Kreipe HH, et al. ADAPTlate: A randomized, controlled, open-label, phase III trial on adjuvant dynamic marker—Adjusted personalized therapy comparing abemaciclib combined with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy in (clinical or genomic) high-risk, HR+/HER2− early breast cancer. J Clin Oncol. 2021;39:TPS598.
Harbeck N, Gluz O, Christgen M, Braun M, Thill M, Wimberger P, et al. Adjuvant dynamic marker-adjusted personalized therapy comparing endocrine therapy plus ribociclib versus chemotherapy in intermediate-risk HR+/HER2− early breast cancer: ADAPTcycle. J Clin Oncol. 2022;40:TPS609.
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–29.
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat. 2017;165:573–83.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
Author Contribution
All authors contributed to the conception and design. The manuscript was written by Anna Fenzl, PhD, and Professor Michael Gnant, MD, FACS, FEBS. All authors read and approved the final manuscript.
Disclosures and declarations
The expert meeting, on which this publication is based, was held in July 2022 in an entirely virtual format, supported by the logo placement of Eli Lilly Austria Ges.m.b.H. without the company having a role in the design, execution, interpretation, or writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M. Gnant reports personal fees/travel support from AstraZeneca, Daiichi Sankyo, Eli Lilly, Menarini-Stemline, MSD, Novartis, PierreFabre, Veracyte; an immediate family member is employed by Sandoz. C.F. Singer reports grants and honoraria by Amgen, Daiichi Sankyo, Eli Lilly, Novartis, AstraZeneca, Pfizer, Roche, Seagen, Gilead, MSD. G. Rinnerthaler reports honoraria by Amgen, Daiichi Sankyo, Eli Lilly, Gilead, Novartis, Pfizer, Roche, Seagen as well as a consulting or advisory Role for Amgen, AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, Merk, MSD, Novartis, Pfizer, Pierre Fabre, Roche. Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo, Eli Lilly, Gilead, Merk, Pfizer, Roche. G. Pfeiler received grants and honoraria from AstraZeneca, Eli Lilly, Amgen, Roche, Gilead, MSD, Seagen, Pfizer, Novartis, UCB. M. Balic reports research funding from AstraZeneca/Daiichi Sankyo, Eli Lilly, Novartis, Roche, Pfizer, Pierre Fabre, Samsung as well as a consulting and advisory role for Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eli Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Samsung, Gilead. Travel, Accommodations, Expenses: Amgen, Astra Zeneca, Eli Lilly, Gilead, MSD, Pfizer, Roche. D. Egle reports personal consulting fees and honoraria from AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Seagen. Support for attending meetings/travel from AstraZeneca. R. Bartsch reports an advisory role at AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Gruenenthal, MSD, Novartis, Pfizer, Pierre-Fabre, Puma, Roche, Seagan; lecture honoraria from AstraZeneca, Daichii Sankyo, Eisai, Eli-Lilly, Gilead, Gruenenthal, MSD, Novartis, Pfizer, Pierre-Fabre, Roche, Seagen; research support from Daiichi Sankyo, MSD, Novartis, Roche.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gnant, M., Singer, C.F., Rinnerthaler, G. et al. Position paper on CDK4/6 inhibitors in early breast cancer. memo 16, 135–144 (2023). https://doi.org/10.1007/s12254-023-00878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-023-00878-1