Summary
Immune checkpoint blockade (ICB) has fundamentally improved the treatment landscape of advanced lung cancer. Improved tolerability and encouraging duration of response in selected patients are some of the advantages of ICB over conventional cytotoxic chemotherapies. However, immune-related adverse events (irAEs) possibly affecting multiple organs pose challenges in diagnosis and management. Checkpoint inhibitor pneumonitis (CIP) is a rare but clinically highly relevant irAE that can significantly impair quality of life and can be potentially life threatening. Since its heterogeneity in clinical and radiographic presentation, diagnosis can be challenging. Treatment usually consists of discontinuing or delaying the administration of ICB. If there is no sufficient recovery with this measure, steroid therapy is indicated. Although the majority of cases improves with this therapy, steroid-refractory CIP can be a therapeutic challenge as there is currently no evidence-based standard treatment. We herein present a short review of literature and a case report of relapsing CIP under steroid treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immune checkpoint blockade (ICB) targeting the programmed death (ligand) 1 (PD(L)1) receptor and cytotoxic T lymphocyte associated protein 4 (CTLA-4) has fundamentally improved the treatment landscape of advanced lung cancer. In advanced non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), ICB has become a fundamental part of first-line therapy, and its application is currently under investigation in the (neo)adjuvant setting in earlier disease stages.
Improved tolerability and encouraging duration of response in selected patients are some of the advantages of ICB over conventional cytotoxic chemotherapies. However, altering the immune response may also come with the cost of immune-related adverse events (irAEs) possibly affecting multiple organs and posing challenges in diagnosis and management.
Checkpoint inhibitor pneumonitis (CIP) is a rare but clinically highly relevant irAE that can significantly impair quality of life and can be potentially life threatening. Overall the development of irAEs is associated with improved ICB efficacy and overall survival (OS) [1, 2]; however, severe CIP was associated with decreased objective response rates (ORR), progression-free survival (PFS) and OS in NSCLC patients treated with anti-PD(L)1 therapy [3].
The incidence of CIP varies between 2 and 5% in clinical trials and meta-analyses, but can be as high as 13–19% according to real-world data [4,5,6]. Patients receiving checkpoint inhibitor combinations or PD-(L)1 blockade have a higher risk of developing CIP than those treated with CTLA‑4 inhibitors only. In addition, the incidence of pneumonitis seems to be increased with PD‑1 blockade compared to PD-L1 blockade as PD-L1 inhibitors do not affect the PD-1:PD1‑2 interaction which is supposed to play a role in mediating immune tolerance in lung tissue [7, 8].
Several patient-specific factors and comorbidities were identified as potential risk factors for the development of CIP. Amongst them are a poor Eastern Cooperative Oncology Group (ECOG) functional status > 2 [3], prior thoracic radiotherapy [9], pre-existing interstitial lung disease (ILD) [3, 9,10,11], ICB combination therapies [9] and/or combinations with EGFR TKIs [12, 13] and squamous NSCLC histology [6].
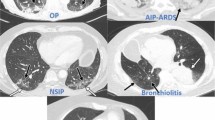
CIP is defined as focal or diffuse inflammation of the lung parenchyma typically accompanied by cough, shortness of breath and hypoxemia but it may be asymptomatic in some cases. Other observed clinical features include fever, chest pain and muscle weakness. The median onset of pneumonitis is 2–3 months after ICB initiation but can vary significantly and may occur earlier under ICB combinations [14]. Radiographic findings are variable and can include nonspecific interstitial pneumonia (NSIP) with ground glass opacities (GGO), cryptogenic organizing pneumonia with predominant consolidations (COP), diffuse alveolar damage or patterns resembling hypersensitivity pneumonia [15]. Different patterns may coincide and localization within the lungs is usually multifocal, involving more than one lobe [6, 15].
Since its heterogeneity in clinical and radiographic presentation, diagnosis can be challenging. Differential diagnoses include tumor progression, infectious diseases, other forms of interstitial lung disease and pneumonitis due to other causes, like medication apart from ICB or radiotherapy [15]. Diagnosis of CIP should be made based on chest-CT imaging, temporal relationship with exposure to the offending drug and exclusion of differential diagnoses, if necessary including lung biopsy, especially to exclude malignancy [15]. Treatment usually consists of discontinuing or delaying the administration of ICB. If there is no sufficient recovery with this measure, high-dose corticosteroids with 1–4 mg/kg/day prednisone are administered until symptoms improve, followed by steroid taper over 4–8 weeks.
Although the majority of cases improves with this therapy, steroid-refractory CIP can be a therapeutic challenge as there is currently no evidence-based standard treatment. Early or repeated disease flare-up after steroid withdrawal can pose a challenge in some patients with CIP that may then resemble cryptogenic organizing pneumonia, where considerably longer corticosteroid therapy and slower dose tapering are recommended [16, 17]. For CIP, other reported therapeutic options in steroid-refractory cases include infliximab, mycophenolate mofetil, intravenous immunoglobulins and the anti-IL‑6 antibody tocilizumab that has been used in selected cases [15, 18,19,20].
Case report
We present a 59-year-old male patient (ex-smoker, 30 pack-years) who presented for the first time in August 2018 after a thoracic trauma with a suspicious lung mass (6 × 5 cm) in the right lower lobe with enlarged hilar and mediastinal lymph nodes. We performed bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration sampling (EBUS-TBNA). He was diagnosed with adenocarcinoma with a PD-L1 TPS > 70%. Molecular analysis also showed a KRAS G12C mutation and a C-met overexpression, without the detection of any other driver mutations. A PET/CT scan revealed metastatic disease with multiple bone lesions. No brain metastases were detected on brain MRI. The patient was staged with T3 N2 M1c – UICC stage IVB disease. There were no relevant comorbidities in the medical history and ECOG functional status was 0, so that the institutional tumor board recommended to initiate a combination chemoimmunotherapy with carboplatin/pemetrexed/pembrolizumab according to the Keynote 189 protocol. In addition, palliative radiotherapy (20 × 2.5 Gy) was applied to a painful bone metastasis located in the right fifth rib. After four cycles of combined chemoimmunotherapy PET/CT showed complete metabolic remission and ICB with pembrolizumab was continued (Fig. 1). After a total of 11 cycles of pembrolizumab, the patient presented with productive cough and in reduced performance status at our clinic. A PET/CT scan showed progressive consolidation in the middle and right lower lobe with subpleural ground glass opacities and progressive pulmonary nodules (Fig. 2a, b). Bronchoscopy was performed, microbiological examination was negative and cytology did not show any tumor cells. The patient was diagnosed with CIP, pembrolizumab treatment was paused and prednisone treatment (1 mg/kg/day) was initiated which resulted in rapid clinical amelioration so that the steroid dose could be gradually tapered and discontinued after 6 weeks. On chest CT scan, the consolidation and GGO had regressed and the pulmonary nodules appeared smaller in size (Fig. 2c). However, only 4 weeks later follow-up imaging again revealed diffuse, patchy infiltrates with tree-in-bud pattern in the left lung basis and the right apex (Fig. 3a–c) and the patient presented with increased symptoms. Hence, steroid treatment was re-introduced. Again, imaging findings and symptoms improved quickly (Fig. 3d–f), allowing steroid tapering again until another relapse only 6 weeks later. Another course of high-dose steroid therapy was initiated and tapering was done over a longer period, which led to long-term improvement. Currently, 3 years after the initial diagnosis of CIP, the patient is on prednisone 2.5 mg/day, without any symptoms or radiographic signs of CIP. Pembrolizumab has not been reintroduced, as there is still no evidence of tumor activity at both the primary site and the metastatic lesions (Fig. 4).
References
Toi Y, Suguwara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–65.
Sato K, Akamatsu H, Muramaki E, et al. Correlation between immune-related adverse events and efficacy in non-small-cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–4.
Tone M, Izuma T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small-cell lung cancer. Thorac Cancer. 2019;10(10):2006–12.
Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12:1607–16.
Fukihara J, Sakamoto K, Koyama J, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442–50. e.4.
Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small-cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9.
Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small-cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–81.
Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211(5):943–59.
Cui P, Liu Z, Wang G, et al. Risk factors for pneumonitis in patients treated with anti-programmed death‑1 therapy: a case-control study. Cancer Med. 2018;7(8):4115–20.
Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small-cell lung cancer. Lung Cancer. 2018;125:150–6.
Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1related pneumonitis in patients with non-small-cell lung cancer: a retrospective analysis. Lung Cancer. 2018;125:212–7.
Oshima Y, Tanimoto T, Yuji K, et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small-cell lung cancer. JAMA Oncol. 2018;4(8):1112–5.
Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–45.
Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17.
Johkoh T, Lee KS, Nishino M, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Radiology. 2021;298(3):550–66.
Epler GR. Bronchiolitis obliterans organizing pneumonia, 25 years: a variety of causes, but what are the treatment options? Expert Rev Respir Med. 2011;5(3):353–61.
Taniguchi H, Kondoh Y. Acute and subacute idiopathic interstitial pneumonias. Respirology. 2016;21(5):810–20.
Ortega Sanchez G, Jahn K, Savic S, et al. Treatment of mycophenolate-resistant immune-related organizing pneumonia with infliximab. J Immunother Cancer. 2018;6(1):85–84.
Petri CR, Patell R, Batalini F, et al. Severe pulmonary toxicity from immune checkpoint inhibitor treated successfully with intravenous immunoglobulin: a case report and review of the literature. Respir Med Case Rep. 2019;27:100834.
Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD‑1 blockade. J Oncol Pharm Pract. 2019;25(3):551–7.
Funding
Open access funding provided by Kepler Universitätsklinikum Linz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. E. Wass, D. Lang, A. Horner and B. Lamprecht declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wass, R.E., Lang, D., Horner, A. et al. Checkpoint inhibitor pneumonitis: Short review of literature and case report. memo 15, 62–66 (2022). https://doi.org/10.1007/s12254-021-00756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-021-00756-8