Summary
The three top abstracts at the 2020 virtual San Antonio Breast Cancer Symposium regarding hormone-receptor-positive early breast cancer, from our point of view, were the long-awaited results from PenelopeB and RxPONDER as well as the data from the ADAPT trial of the West German Study Group. PenelopeB failed to show any benefit by adjuvant palbociclib when added to standard endocrine therapy in patients without pathologic complete response after neoadjuvant chemotherapy. RxPONDER demonstrated that postmenopausal patients with early hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2−) breast cancer, 1–3 positive lymph nodes and an Oncotype DX Recurrence Score of less than 26 can safely be treated with endocrine therapy alone. In contrast, in premenopausal women with positive nodes, adjuvant chemotherapy plays still a role even in case of low genomic risk. Whether the benefit by chemotherapy is mainly an indirect endocrine effect and if ovarian function suppression would be similarly effective, is still a matter of debate. The HR+/HER2− part of the ADAPT umbrella trial investigated the role of a Ki-67 response to a short endocrine therapy before surgery in addition to Oncotype DX—performed on the pretreatment biopsy—to identify low-risk patients who can safely forgo adjuvant chemotherapy irrespective of menopausal status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Review
Within this short review we discuss the, from our point of view, three top abstracts at the 2020 virtual San Antonio Breast Cancer Symposium regarding hormone-receptor-positive (HR+)/HER2 negative (HER2−) early breast cancer (EBC).
PenelopeB trial: endocrine therapy +/− palbociclib as postneoadjuvant therapy for high-risk early breast cancer [10]
Two phase III trials investigating CDK4/6 inhibitors as adjuvant therapy in HR+/HER2− EBC were already presented at the 2020 virtual congress of the European Society of Medical Oncology (ESMO) [1, 2]. The PALLAS trial investigated a 2-year treatment with the first-in-class CDK4/6 inhibitor palbociclib in combination with endocrine therapy in 5760 patients with HR+/HER2− stage II and III breast cancer. Compared to endocrine therapy alone, palbociclib did not prolong invasive disease-free survival (iDFS: hazard ratio [HR] 0.93; 95% confidence interval [CI] 0.76–1.15; P = 0.51) [2]. In contrast, the MonarchE trial, including only lymph-node-positive patients, showed a statistically significant benefit in iDFS from a 2-year therapy with abemaciclib in combination with endocrine therapy compared to endocrine therapy alone. The difference in iDFS was already statistically significant at the ESMO presentation of the second interim analysis, despite a very short follow-up of 15.5 months. At the 2020 San Antonio Breast Cancer Symposium the primary outcome iDFS analysis of MonarchE was presented with a slightly longer median follow-up of 19.1 months. A risk reduction for an iDFS event of 28.7% was shown by the addition of abemaciclib (HR 0.71; 95% CI 0.58–0.87; P < 0.001), resulting in an absolute iDFS gain of 3.0% at year two (92.3 vs. 89.3%) [3]. At data cut-off, however, 58% of patients were still on treatment and only 26% completed the 2‑year treatment period.
Considering the conflicting PALLAS and MonarchE results, the first results from the PenelopeB trial, presented at the virtual SABCS 2020, were eagerly awaited. In contrast to the former two trials, the double-blind, placebo-controlled, phase III PenelopeB study recruited only HR+/HER2− breast cancer patients without pathologic complete remission (pCR) after neoadjuvant chemotherapy. To select patients with a high risk for recurrence, patients had to have a CPS-EG (pretreatment Clinical stage, posttreatment Pathologic Stage, Estrogen receptor status, nuclear Grade) score [4] of greater than 3 or—in case of persistent nodal-positivity—a score of greater than two. Patients were randomized 1:1 to palbociclib at standard dose for 1 year or to placebo for the same length of time. All patients were treated with endocrine therapy according to local standards. The median follow-up of PenelopeB at the data cut-off was 42.8 months, which is considerably longer than those of PALLAS (23.7 months) or MonacheE (19.1 months). Even though patient adherence was much better than in PALLAS (20% early discontinuations compared to 42%), PenelopeB could not demonstrate a statistically significant benefit in iDFS (HR 0.93; 95% CI 0.74–1.17; P = 0.525; Fig. 1). Interestingly, at year 2 and 3, the iDFS rate was meaningfully higher in the palbociclib arm (88.3 vs. 84.0% and 81.2 vs. 77.7%, respectively); however these advantages disappeared at year 4 (73.0 vs. 72.4%). In subgroup analysis, no patient group could be identified which seemed to have a benefit from the CDK4/6 inhibition. In addition, the types of iDFS events were similar between the two treatment arms with distant recurrences representing 74% of all events. Similar to iDFS, overall survival (OS) was not significantly different between the palbociclib and the placebo arm (HR 0.87; 95% CI 0.61–1.22; P = 0.420). Palbociclib was generally well tolerated with a known toxicity profile of mainly hematologic adverse effects (73% G3/4 neutropenia).
Invasive disease-free survival (iDFS) of the PenelopeB trial at a median follow-up of 42.8 months [10]. With kind permission from S. Loibl. This figure is not included under the Creative Commons CC BY license of this publication
These results demonstrate the importance of a sufficient follow-up time for the final interpretation of adjuvant trials. The temporary separation of the survival curves of the PenelopeB trial in the first 3 years suggests that longer treatment with palbociclib may be beneficial. This question is partly addressed by the ongoing NATALEE trial, investigating 3 years of ribociclib.
RxPONDER trial: Oncotype DX in patients with 1–3 positive lymph nodes [11]
The TAILORx trial demonstrated that patients with node negative early HR+/HER2− breast cancer and an Oncotype DX Recurrence Score (RS) of < 26 have generally an excellent prognosis with no benefit from adjuvant chemotherapy [5, 6]. The only exception from this finding were patients 50 years or younger with a RS of 21–25, who seemed to have a risk reduction for recurrence by adjuvant chemotherapy (−5.8% at 5 years) [7].
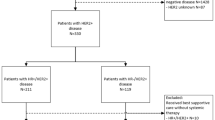
Based on these findings, the results of the RxPONDER trial, which included only patients with 1–3 positive lymph nodes, were long-awaited. Patients fit enough for adjuvant chemotherapy and a RS of 0–25 were randomized between chemotherapy followed by endocrine therapy and endocrine therapy alone. Patients were stratified by RS (0–14 vs. 15–25), menopausal status (pre- vs. postmenopausal), and type of axillary surgery (lymph node dissection vs. sentinel node biopsy). The primary endpoint was iDFS, secondary endpoints were OS, distant and locoregional DFS.
The patient characteristics were well balanced between the two arms: 33% were premenopausal, 66% had only one lymph node involved, 9% three positive lymph nodes. In the chemotherapy arm, 50% received 4–6 cycles of docetaxel/cyclophosphamide and only 3% of premenopausal women received ovarian function suppression (OFS). In the total population a small but statistically significant benefit from adjuvant chemotherapy was detected with an absolute iDFS benefit after 5 years of 1.4% (HR 0.81; 95% CI 0.67–0.98; P = 0.026). This positive effect, however, was restricted to premenopausal women, who derived an absolute benefit after 5 years of 5.2% (HR 0.54; 95% CI 0.38–0.76; P = 0.0004; Fig. 2). In this population OS was also statistically significant better in the chemotherapy arm (HR 0.47; 95% CI 0.24–0.94; P = 0.032).
a Postmenopausal women with early HR+/HER2− breast cancer with 1–3 positive nodes did not benefit from adjuvant chemotherapy if the Oncotype DX Recurrence Score was < 26. b Premenopausal women had a statistically significant better invasive disease-free survival if adjuvant chemotherapy was given [11]. With kind permission from K. M. Kalinsky. This figure is not included under the Creative Commons CC BY license of this publication
Hence, the benefit from adjuvant chemotherapy in premenopausal women was reproduced in RxPONDER. Similar results were also observed in an exploratory analysis of the MINDACT trial were an absolute chemotherapy benefit in distant metastasis-free survival (DMFS) of 5.0% was seen after 8 years of follow-up in patients ≤ 50 years with high clinical risk but low genomic risk according to MammaPrint [8]. In all of these three trials the rate of patients receiving OFS was low (between 3 and 16% in the different treatment arms) it is possible that this age dependent effect is a result of chemotherapy-induced OFS rather than of direct cytotoxicity. The role of adjuvant chemotherapy in premenopausal patients treated with OFS is therefore still a matter of debate and cannot be answered by these trials.
RxPONDER and TAILORx show clearly that postmenopausal patients with early HR+/HER2− breast cancer, 0–3 positive lymph nodes and an Oncotype DX RS of 0–25 can safely be treated by endocrine therapy alone. Premenopausal women with a RS 16–25 or positive lymph nodes, tamoxifen monotherapy should definitely be avoided and either chemotherapy be added or OFS in combination with an aromatase inhibitor be used.
WSG-ADAPT HR+/HER2: endocrine monotherapy in patients with RS < 12 or RS 12–25 and Ki-67 response after 3 weeks of endocrine therapy [12]
The ADAPT trial of the West German Study Group is a large umbrella trial including different breast cancer subtypes. In the endocrine part of the trial—similar to RxPONDER and TAILORx—Oncotype DX was used for primary risk stratification in patients with HR+/HER2− early breast cancer and a general indication for adjuvant chemotherapy (cT2 or G3 or Ki-67 ≥ 15% or N+). In contrast to the other trials, in ADAPT all patients received 3 weeks of endocrine therapy (tamoxifen or an aromatase inhibitor) between the diagnostic biopsy and surgery. The diagnostic biopsy was used to determine Oncotype DX RS and Ki-67. At surgery Ki-67 was retested and in case of a RS of 12–25 only patients with a Ki-67 response (defined as Ki-67 < 10% at surgery) were included in the trial. All patients received endocrine monotherapy according to the investigator. The primary goal of the trial was to show that patients with a RS 12–25 and a Ki-67 response do not have a lower iDFS at 5 years than patients with a RS < 12. As assumed, the 5‑year iDFS was noninferior (93.9 vs. 92.6%; P = 0.05). Similarly, the distant disease-free survival (dDFS) and OS were also comparable between the two groups. There was also no difference in dDFS in patients ≤ 50 years (Fig. 3) or with positive lymph nodes. Only patients with a RS 12–25 and three or more involved lymph nodes had a numerically lower dDFS compared to patients with a RS < 12. These patients may therefore not be ideal candidates for endocrine therapy alone.
Distant disease-free survival (dDFS) at year 5 was not different between patients with RS 12–25 but Ki-67 response after 3 weeks of endocrine therapy and patients with a RS < 12 both in patients below and above 50 years [12]. With kind permission from N. Harbeck. This figure is not included under the Creative Commons CC BY license of this publication
In summary, the WSG-ADAPT HR+/HER2 trial identified a subgroup of premenopausal women (Ki-67 after 3 weeks of endocrine therapy < 10%), whom adjuvant chemotherapy could be safely spared. This finding, however, is based on a total of only 330 patients and therefore these results have to be interpreted with caution. Still open is the question, how patients with inadequate Ki-67-drop after 3 weeks of endocrine therapy should be ideally treated. The results of the ALTERNATE trial [9] suggest that switching to chemotherapy is not enough (pCR rate of only 4.8%). Whether the addition of a CDK4/6 inhibitor is more beneficial is currently being investigated in the ADAPTcycle trial (NCT04055493).
Conclusion
Because of the negative results of PALLAS and PenelopeB, MonarchE remains currently the only positive phase III trial investigating adjuvant CDK4/6 inhibitors in early HR+/HER2− breast cancer. Longer follow-up is needed before 2 years of abemaciclib can be declared as new standard of care for lymph-node-positive, high-risk HR+/HER2− breast cancer. The results of the NATALEE trial investigating 3 years of ribociclib are still awaited.
The data of RxPONDER clearly strengthened the role of Oncotype DX in risk prediction and the choice of adjuvant chemotherapy for HR+/HER2− breast cancer. Postmenopausal patients with less than 4 positive lymph nodes should only receive adjuvant chemotherapy if the RS is above 25. In premenopausal women with positive nodes, adjuvant chemotherapy plays still a role even in case of low genomic risk. Maybe a Ki-67 response to a short preoperative endocrine therapy could help to identify patients with an intermediate risk according to Oncotype DX, who can safely forgo adjuvant chemotherapy.
References
Johnston SRD, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–98.
Mayer EL, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–22.
Shaughnessy JA, et al. Abstract GS1-01: Primary outcome analysis of invasive disease-free survival for monarchE: abemaciclib combined with adjuvant endocrine therapy for high risk early breast cancer. Cancer Res. 2021;81(4 Suppl):GS1-01.
Mittendorf EA, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29(15):1956–62.
Sparano JA, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
Sparano JA, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–14.
Sparano JA, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–405.
Piccart M, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22(4):476–88.
Ma CX, et al. Abstract GS4-05: Neoadjuvant chemotherapy (NCT) response in postmenopausal women with clinical stage II or III estrogen receptor positive (ER+) and HER2 negative (HER2−) breast cancer (BC) resistant to endocrine therapy (ET) in the ALTERNATE trial (Alliance A011106). Cancer Res. 2021;81(4 Suppl):GS4-05.
Loibl S, et al. Abstract GS1-02: Phase III study of palbociclib combined with endocrine therapy (ET) in patients with hormone-receptor-positive (HR+), HER2-negative primary breast cancerand with high relapse risk after neoadjuvant chemotherapy (NACT): First results from PENELOPE-B. Cancer Res. 2021;81(4 Suppl):GS1-02.
Kalinsky K, et al. Abstract GS3-00: First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy (ET) +/- chemotherapy (CT) in patients (pts) with 1–3 positive nodes, hormone receptor-positive (HR+) and HER2-negative (HER2–) breast cancer (BC) with recurrence score (RS) < 25: SWOG S1007 (RxPonder). Cancer Res. 2021;81(4 Suppl):GS3-00.
Harbeck N, et al. Abstract GS4-04: Endocrine therapy alone in patients with intermediate or high-risk luminal early breast cancer (0–3 lymph nodes), Recurrence Score < 26 and Ki67 response after preoperative endocrine therapy: Primary outcome results from the WSG-ADAPT HR+/HER2– trial. Cancer Res. 2021;81(4 Suppl):GS4-04.
Funding
Open access funding provided by Paracelsus Medical University.
Author information
Authors and Affiliations
Contributions
Conception and design: S. P. Gampenrieder; collection and assembly of data: S. P. Gampenrieder; cata analysis and interpretation: all authors; manuscript writing: S. P. Gampenrieder and G. Rinnerthaler; critical revising of the manuscript: G. Rinnerthaler, R. Greil; final approval of manuscript: all authors
Corresponding author
Ethics declarations
Conflict of interest
Employment or Leadership Position: None; Consultant or Advisory Role: S. P. Gampenrieder for Pfizer, Novartis, Eli Lilly; G. Rinnerthaler for Pfizer, Novartis, Eli Lilly; R. Greil for Novartis; Stock Ownership: None; Speakers Honoraria: S. P. Gampenrieder from Novartis; Rinnerthaler from Novartis; R. Greil from Novartis; Travel Grants: S. P. Gampenrieder from, Novartis, Pfizer; G. Rinnerthaler from Novartis, Pfizer; Research Funding: R. Greil from Novartis (no personal payments).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gampenrieder, S.P., Rinnerthaler, G. & Greil, R. Top 3 abstracts concerning hormone-receptor-positive early breast cancer. memo 14, 252–256 (2021). https://doi.org/10.1007/s12254-021-00726-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-021-00726-0