Summary
At the ESMO (European Society for Medical Oncology) 2020 several interesting albeit not practice-changing studies in the field of pancreatic cancer were presented. The Canadian phase II randomized PA.7 trial investigated the additional benefit of dual checkpoint inhibition with durvalumab and tremelimumab to a standard chemotherapy regimen as first-line treatment in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). Unfortunately, no significant improvement of responses or outcome could be achieved rendering this study a negative trial. Within the German platform-based QoliXane trial, quality of life was shown to be an essential prognosticator of survival with fatigue and nausea being independently associated with outcome of patients. Moreover, promising results could be observed with new targeted therapy approaches, which may lead to its investigation in larger randomized clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immunotherapy in pancreatic cancer
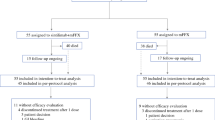
Targeting immune checkpoints by monoclonal antibodies has led to a tremendous treatment change and improvement of prognosis of cancer patients over the last decades. Pancreatic cancer in contrast is characterized by a highly immunosuppressive microenvironment with a low tumor mutational burden and a lack of effector T‑cells [1]. Only 1% of metastatic pancreatic ductal adenocarcinoma (mPDAC) patients harbor DNA mismatch repair-deficient (MMR-D) tumors potentially representing the most immunogenic subtype. Preclinical data so far suggest a synergistic effect of combining chemotherapy with immunotherapies in pancreatic cancer models [2]. Within the currently largest non-randomized single-arm trial investigating the combination of gemcitabine, nab-paclitaxel and the anti-PD‑1 antibody nivolumab as first-line therapy, objective responses could be observed in 18% of patients and a stabilization of disease in 46% of mPDAC patients [3]. However, median overall survival (OS) remained limited with 9.9 months. A dual immunotherapeutic approach was first investigated in 2019 within a phase II randomized clinical trial testing the combination of the anti-PD-L1 inhibitor durvalumab and the anti-CTLA4-inhibitor tremelimumab applied as second-line treatment [4]. Here, no additional benefit with the combination compared to durvalumab alone could be observed with a median OS of only 3.1 months. Presented at the ESMO 2020, the phase II trial PA.7 of the Canadian Cancer Trials Group first investigated the efficacy and safety of a dual checkpoint blockade combined with a standard chemotherapy regimen applied as first-line treatment of mPDAC patients [5]. Randomized in a 2:1 ratio, 180 patients received either a combination of gemcitabine (1000 mg/m2, day 1, 8, 15, q28), nab-paclitaxel (125 mg/m2, day 1, 8, 15, q28), durvalumab (1500 mg, day 1, q28) and tremelimumab (75 mg, q28) or gemcitabine and nab-paclitaxel alone. Despite an increased response and disease control rate within the interventional arm, median progression-free survival (PFS; 5.5 months in the interventional arm vs 5.4 months in the standard arm) as well as OS (9.8 months in the interventional arm vs 8.8 months in the standard arm) was not significantly different. Interestingly, the addition of the two immunotherapies did not result in any meaningful increase in grade 3/4 events compared to the standard chemotherapy regimen. In conclusion, no benefit could be observed in the overall patient population with the addition of immunotherapy. However, circulating tumor DNA (ctDNA) analyses conducted within this study may help to assess immune sensitivity in this setting. A summary of previously conducted trials investigating the combination of chemotherapy with immunotherapy in mPDAC patients is listed in Table 1. Several trials addressing this topic are currently ongoing (e.g., NCT03829501, NCT01928394).
Quality of life in metastatic disease
Pancreatic cancer is known to cause a considerable high symptom burden compared to other tumor entities. Therefore, the assessment of quality of life (QoL) within prospective randomized trials seems of major interest to support daily treatment decisions. Supposedly, efficient disease control achieved by a chemotherapy regimen prevents deterioration of QoL and therefore outweighs its potential toxicities [6, 7]. In line with this, disease progression is usually associated with a deterioration of QoL [8]. The QoliXane trial presented at the ESMO 2020 is a prospective, multicenter study accessing QoL based on a platform for outcome, QoL and translational research on pancreatic cancer (Paragon) under the frontline chemotherapy combination of gemcitabine and nab-paclitaxel [9]. A total of 600 patients were included and EORTC-C30 questionnaires for the assessment of QoL were collected at baseline, 3 and 6 months respectively after initiation of treatment. They reported a median PFS of 5.9 months and a median OS of 8.9 months in line with the phase III MPACT trial investigating the combination of nab-paclitaxel and gemcitabine as first-line treatment of mPDAC patients [10]. QoL measured by the Global Health score (GHS) at baseline in general significantly predicted survival. Median time to deterioration was fairly short with only 4.7 months. Nevertheless, 61% of mPDAC patients presented with a maintained QoL after 3 months and 41% of patients after 6 months. Almost all side effects were significantly associated with OS, but only fatigue as well as nausea and vomiting remained independent prognosticators. An increase in physical function was significantly associated with a decreased risk of death. This study therefore highlights the importance of disease control and optimization of symptom burden in mPDAC patients.
New targeted therapy approaches
Beside many ongoing studies, the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib remains the only approved targeted therapy for mPDAC patients with a germline breast cancer (BRCA) mutation to date. Prospective and predictive biomarkers are urgently needed to identify new targeted therapy approaches. Dean et al. investigated the combination of gemcitabine and nab-paclitaxel with the tumor-penetrating peptide CEND1 as first-line treatment of metastatic disease [11]. By interaction with neuropilin‑1, this targeted agent transforms the tumor microenvironment into a conduit potentially leading to increased local efficacy of the combined chemotherapy agents. Within their study, they observed an indeed promising overall response rate of 59% and stable diseases in 34% of patients. Albeit single-arm, observed responses were much more pronounced compared to previously reported studies of the chemotherapy regimen alone [10].
Halama et al. investigated the safety and efficacy of NOX-A12, which targets the chemokine protein CXCL12 and thereby modulates the tumor microenvironment, in combination with the PD-1-inhibitor pembrolizumab in heavily pretreated microsatellite stable metastatic colorectal cancer and mPDAC patients [12]. A tumor biopsy before and after monotherapy with this targeted agent revealed an enhanced effector immune cell infiltration of the tumor and may thereafter lead to a more effective immune response caused by immunotherapy. Albeit not separately analyzed, stable disease could be achieved in 25% of all treated patients after a median of three previously applied therapy lines. Future investigation of prognostic and predictive biomarkers is urgently needed to identify potential therapeutic targets.
Take home message
-
The addition of a dual checkpoint blockade with durvalumab and tremelimumab to standard chemotherapy failed to improve response rates and outcome of metastatic pancreatic ductal adenocarcinoma (mPDAC) patients.
-
Quality of life is predictive for the prognosis of mPDAC patients under first-line treatment and therefore toxicity management needs to be optimized.
-
New targeted therapy approaches like tumor-penetrating peptide CEND1 or NOX-A12 need to be investigated in larger clinical trials.
References
Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–36. https://doi.org/10.1158/1078-0432.CCR-17-3099.
Winograd R, Byrne KT, Evans RA, et al. Induction of T‑cell immunity overcomes complete resistance to PD‑1 and CTLA‑4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3(4):399–411. https://doi.org/10.1158/2326-6066.CIR-14-0215.
Wainberg ZA, Hochster HS, Kim EJ, et al. Open-label, phase I study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4814–22. https://doi.org/10.1158/1078-0432.CCR-20-0099.
O’Reilly EM, Oh D‑Y, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1431–8. https://doi.org/10.1001/jamaoncol.2019.1588.
Renouf DJ, Knox JJ, Kavan P, et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab‑P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcino. Ann Oncol. 2020;31:1195. https://doi.org/10.1016/j.annonc.2020.08.2300.
Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31(1):23–9. https://doi.org/10.1200/JCO.2012.44.4869.
Hubner RA, Cubillo A, Blanc J‑F, et al. Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5‑fluorouracil and leucovorin. Eur J Cancer. 2019;106:24–33. https://doi.org/10.1016/j.ejca.2018.09.029.
Hammel P, Kindler HL, Reni M, et al. Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Ann Oncol. 2019;30(12):1959–68. https://doi.org/10.1093/annonc/mdz406.
Al-Batran S‑E, Hofheinz RD, Reichart A, et al. Real-life results from the prospective QoliXane trial of the platform for outcome, quality of life, and translational research on pancreatic cancer (PARAGON) registry. J Clin Oncol. 2020;38(15_suppl):4625. https://doi.org/10.1200/JCO.2020.38.15_suppl.4625.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. https://doi.org/10.1056/NEJMoa1304369.
Dean A, Gill S, McGregor M, Broadbridge V, Jarvelainen HA, Price TJ. 1528P Phase I trial of the first-in-class agent CEND‑1 in combination with gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer. Ann Oncol. 2020;31:941. https://doi.org/10.1016/j.annonc.2020.08.2011.
Halama N, Williams A, Suarez-Carmona M, et al. 1537P Phase I/II study with CXCL12 inhibitor NOX-A12 and pembrolizumab in patients with microsatellite-stable, metastatic colorectal or pancreatic cancer. Ann Oncol. 2020;31:944. https://doi.org/10.1016/j.annonc.2020.08.2020.
Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25(9):1750–5. https://doi.org/10.1093/annonc/mdu205.
Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36(1):96–102. https://doi.org/10.1007/s10637-017-0525-1.
O’Hara MH, O’Reilly EM, Rosemarie M, et al. Abstract CT004: a phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res. 2019; https://doi.org/10.1158/1538-7445.AM2019-CT004.
Kamath SD, Kalyan A, Kircher S, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist. 2020;25(5):e808–e15. https://doi.org/10.1634/theoncologist.2019-0473.
Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26(6):878–85. https://doi.org/10.1038/s41591-020-0880-x.
Funding
Open access funding provided by Medical University of Vienna.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.S. Bergen declares that she has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergen, E.S. ESMO 2020 update: Pancreatic cancer. memo 14, 176–179 (2021). https://doi.org/10.1007/s12254-021-00692-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-021-00692-7