Summary
The intestinal microbiota seems to play a key role in many gastrointestinal, pancreatic and liver disorders. Dysbiosis, a substantial alteration in the intestinal microbiome, is associated with chronic liver disease (CLD) compared to healthy individuals. These findings were shown in several preclinical and clinical studies and were most distinct in the stage of cirrhosis. The pathogenesis of hepatocellular carcinoma (HCC) and its underlying diseases is still not completely understood: Bacteria and related metabolites and pro-inflammatory signals may be involved. Several animal and human studies have focused on the role of intestinal microbiota in HCC. Here a key role of the intestinal microbiota in the pathogenesis could be addressed, whereby the abundance of pro-inflammatory intestinal species is increased. Additionally, some studies could demonstrate a decrease of butyrate-producing species and other species known for their anti-inflammatory potential. Furthermore, multiple preclinical studies could demonstrate that the intestinal microbiota is a key player in hepatocarcinogenesis. The intestinal microbiota seems to interact with the central pathways of hepatocarcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is an aggressive malignancy developing from underlying chronic liver disease (CLD). HCC has become the third leading cause of cancer mortality worldwide [1]. Underlying diseases differ between low- and high-income countries. While viral hepatitis is the leading cause in low-income countries, alcoholic (ALD) and nonalcoholic (NAFLD) fatty liver disease represent the most frequent cause in the western hemisphere [2]. The pathogenesis is diverse, driven by a vicious circle of liver injury, inflammation and regeneration.
The understanding of genetically associated HCC development changed completely through genome wide association studies (GWAS). Useful suggestions on signaling pathway of tumor development were discovered through these genetic associations [3]. But these studies could not clarify the functional understanding of HCC. The current model of disease is a combination of predisposing factors and genetic risk. The bacterial microbiome seems to play a key role in promoting liver diseases and the development of HCC and evidence still increases [4].
The liver is closely linked to the gut, due to its anatomical connection via the portal vein. The liver receives nutrient-enriched blood from the intestine; it is also the first target of the intestinal microbiota and microbe-associated molecular patterns (MAMPs). MAMPs can trigger inflammatory responses via pattern-recognition receptors (PRRs) and microbial metabolites [5]. In healthy individuals, the hepatic exposure to pro-inflammatory MAMPs is limited due to the multilayer intestinal barrier. Alterations of the gut microbiota in CLD lead to a failing gut barrier via chronic inflammation. This process is a key mechanism leading to the progression of liver diseases and inferentially increases the risk of HCC development [6,7,8].
Bacterial dysbiosis in liver cirrhosis
Increasing evidence points to a key role of the gut microbiota in the development of CLD [9]. There are several animal studies showing a clear association between CLD and microbial dysbiosis. These findings could be confirmed by human data. There is strong evidence that the progression of NAFLD, ALD and chronic viral hepatitis is strongly associated with gut microbiome dysbiosis. In patients suffering from HBV and HCV, microbial diversity is decreased in comparison to health controls [10]. The complex network between NAFLD and dysbiosis is highlighted in multiple studies [9, 11, 12] and is also seen in patients suffering from ALD—microbiome alterations were associated with decreased levels of butyrate-producing Clostridiales species and increased levels of pro-inflammatory Enterobacteriaceae. Bacteroidales were reduced in patients diagnosed with cirrhosis [13]. Enterococcus faecalis is strongly correlated with the severity of liver disease and with mortality in patients suffering from alcoholic hepatitis [14]. In one study, bacteriophages targeting cytolytic Enterococcus faecalis, abolished induced liver disease in humanized mice [15]. Phages might therefore be a novel therapeutic approach in treating patients with alcoholic liver disease.
Furthermore, the microbiome influences progression from pre-existing diseases into cirrhosis, which emphasizes the central role for dysbiosis in the development of end stage liver disease in murine models as well as in human patients [16, 17].
Patients with advanced liver disease and cirrhosis have an alteration in the gut microbiome. These alterations are associated with an increase of potentially pathogenic bacteria and reduced numbers of bacteria with beneficial properties [18].
The composition of the intestinal microbiota in cirrhosis include enrichment of Veillonella or Streptococcus, as well as decreased numbers from the order Clostridiales [18]. In another study, in patients suffering from cirrhosis, the gut microbiome presented with a relative reduction in Bacteroidetes, an increase in Proteobacteria and Fusobacteria. Changes in Firmicutes mimicked the microbiome from healthy individuals [19]. Furthermore, there were differences at the family level, with Streptococcaceae and Veillonellaceae. Streptococcaceae positively correlated with cirrhosis severity, while Lachnospiraceae negatively correlated with disease activity. These differences were confirmed by another research group in a larger population of patients suffering from cirrhosis [19, 20].
An invasion of the gut from the mouth in patients with liver cirrhosis is most likely: the majority of enriched species were of buccal origin [18]. Furthermore, the abundance of buccal origin species in cirrhosis is associated with the use of proton pump inhibitors (PPIs). PPIs modulate microbiota composition in patients with CLD [21]. Besides alterations in the gut microbiome, bacterial overgrowth in the upper gastrointestinal tract, which is associated with increased circulating lipopolysaccharide (LPS) levels, seems to play a major role in the development of CLD [22]. Due to the close anatomic position of the small intestine to the liver, bacterial translocation in the upper gastrointerstinal tract is important in the pathogenesis of liver disease. Differences in the duodenal and salivary microbiota between patients suffering from cirrhosis and healthy individuals have been demonstrated in different studies. Qualitative and quantitative changes in the upper gastrointestinal tract might be linked to changes in the more distal microbiota. It is most likely that these findings point out that microbial changes in the upper gastrointestinal tract contribute to the complex pathophysiology of CLD as well as in the development of HCC [23].
Alteration in the microbiome in patients suffering from CLD are not only established in feces, but also in serum, saliva, sigmoid colonic mucosa, small intestinal mucosa, ascites and liver tissue [19]. Furthermore, dysbiosis is connected to main comorbidities of cirrhosis, including hepatic encephalopathy, spontaneous bacterial peritonitis, and multiple organ failure leading to death [20]. This evidence supports the conclusion that dysbiosis in cirrhosis causes holistic mucosal immune change and vice versa. Furthermore, emerging evidence also points towards the important roles of archaea, viruses, fungi and especially bacteriophages [24]. Further studies are needed to investigate and define the holistic changes in the microbiome in liver diseases.
HCC and microbiota
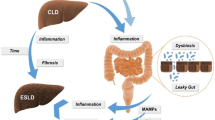
Dysbiosis plays a key role in the development of liver disease and HCC. In mouse models and humans with CLD and HCC high circulation LPS-levels were demonstrated, implicating the presence of an impaired intestinal barrier during multiple stages of CLD and in hepatocarcinogenesis [25, 26]. In animal models an impaired intestinal barrier played a key role in hepatocarcinogenesis via LPS and its receptor toll like receptor (TLR) 4 [17]. “Leaky gut” is associated with increased bacterial translocation and generates a chronic inflammatory state in the liver. Hepatocarcinogenesis via LPS resulting from an impaired intestinal barrier is driven via multiple cellular targets including HSCs, the hepatocyte-tumor compartment and Kupffer cells. The inflammatory responses in the liver are mediated via interaction between MAMPs and host PRRs, specifically the TLRs ([27]; Fig. 1). These pathways of pathogenesis have been demonstrated in several animal models. Only a few clinical trials correlating microbiota and HCC are published and show different alterations of the gut microbiota in patients with HCC. An increase in fecal Eschericha coli was associated with the presence of HCC in cirrhotic patients, suggesting that an intestinal enrichment of Eschericha coli could contribute to the process of hepatocarcinogenesis [28]. Results were confirmed in another study. In HCC patients suffering from hepatitis B or hepatitis C as underlying disease, more potential pro-inflammatory bacteria (Escherichia, Shigella, Enterococcus) and reduced levels of Faecalibacterium, Ruminococcus, and Ruminoclostridium were observed and results in a decrease of potentially anti-inflammatory short-chain fatty acids [29]. In another study, patients with NAFLD-related cirrhosis and HCC, NAFLD-related cirrhosis without HCC, and healthy controls were compared. An increase of pro-inflammatory cytokines in the plasma of patients with HCC compared those without HCC was detected. The fecal microbiota of the cirrhosis group showed higher abundance of Enterobacteriaceae and Streptococcus and a reduction in Akkermansia. Bacteroides and Ruminococcaceae were increased in the HCC group, while Bifidobacterium was reduced [8]. In a study of early HCC, patients with or without cirrhosis where compared. Microbial diversity was significantly increased in HCC patients without signs of cirrhosis. The phylum Actinobacteria was increased in early HCC compared to patients with liver cirrhosis. In early HCC, thirteen genera including Gemmiger and Parabacteroides were enriched versus HCC with cirrhosis. Furthermore, LPS producing species Parabacteroides, were increased and the butyrate-producing species Actinobacteriae were decreased in patients with HCC compared to healthy individuals. Current evidence suggests that a specific microbiota pattern for patients with HCC does exist [30].
Contribution of the gut microbiota to hepatocarcinogenesis. Progression of liver disease and development of hepatocellular carcinoma (HCC) is modulated by dysbiosis and impaired intestinal barrier via multiple different mechanisms. The dysbiotic microbiota promotes cancer-promoting metabolites like lipopolysaccharides (LPS), short chain fatty acids (SCFAs), and bile acids that boost hepatic inflammation and inhibition of apoptosis. An increased hepatic exposure to gut-derived microbe-associated molecular patterns (MAMPS) hepatic inflammation, fibrosis and inhibition of apoptosis as well
Conclusion
The intestinal microbiota and its alterations are strongly associated with CLD and the development of HCC. The alterations in intestinal microbiota create a pro-inflammatory hepatic milieu. The mechanisms and interactions leading to hepatic inflammation are diverse. First, dysbiosis leads to an impaired intestinal barrier that enhances TLR-mediated chronic liver inflammation and, second, altered and potentially pro-inflammatory bacterial metabolites are promoted by an impaired intestinal barrier. Whether altered bacterial metabolites or chronic inflammation are associated with the translocation of MAMPs from the impaired intestinal barrier are key players in hepatocarcinogenesis is subject of current research. It is highly suggestive that the impaired gut barrier and the dysbiosis act synergistically in the development of HCC. Furthermore, HCC might be associated with a specific gut microbiome signature. The current understanding of the interactions between dysbiosis, chronic liver disease and HCC are mainly due to preclinical studies and fecal samples from patients suffering from liver diseases. There is a tremendous need for human microbiome studies to substantiate the key role of the intestinal microbiome in the pathogenesis of HCC. Such studies could lead to a potential therapy for this highly lethal human disease.
Take home massage
The intestinal microbiota is a key player in hepatocarcinogenesis. Influencing the microbiota could lead to potential novel treatment strategies in this highly lethal disease.
References
Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846.
Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(Suppl 3):S206–S14.
Cancer Genome Atlas Research Network. Electronic address wbe, cancer genome Atlas research N. comprehensive and integrative genomic characterization of Hepatocellular carcinoma. Cell. 2017;169(7):1327–1341e23.
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411.
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–24.
Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–16.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101.
Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69(1):107–20.
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65(12):2035–44.
Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6:34055.
Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9.
Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25.
Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5(1):141.
Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–6.
Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575(7783):505–11.
De Minicis S, Rychlicki C, Agostinelli L, Saccomanno S, Candelaresi C, Trozzi L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59(5):1738–49.
Acharya C, Sahingur SE, Bajaj JS. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017; https://doi.org/10.1007/s12254-020-00597-x.
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64.
Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72.
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–7.
Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol. 2018;113(8):1177–86.
Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, Aponte JJ, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97(9):2364–70.
Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62(4):1260–71.
Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51.
Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22(2):165–72.
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57(4):803–12.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20.
Grat M, Wronka KM, Krasnodebski M, Masior L, Lewandowski Z, Kosinska I, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant Proc. 2016;48(5):1687–91.
Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, et al. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1.
Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2018; https://doi.org/10.1136/gutjnl-2017-315084.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Effenberger and H. Tilg declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Effenberger, M., Tilg, H. The intestinal microbiota and hepatocellular carcinoma. memo 13, 223–226 (2020). https://doi.org/10.1007/s12254-020-00597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-020-00597-x