Summary
This short review summarizes the most important facts presented at the San Antonio Breast Cancer Symposium 2018 concerning local therapy of breast cancer, including breast and lymph node surgery as well as radiotherapy. Despite the increasing use of neoadjuvant chemotherapy (nCHT) and consequently higher response and pathologic complete response (pCR) rates in the past years, breast-conserving surgery (BCS) has not increased. This is not only due to positive genetic testing, but it is mostly based on patients’ preference. On the other hand, quality of life seems to be better in young patients undergoing BCS regarding satisfaction with breasts and sexual and psychosocial well-being. Thus, surgical decision-making is crucial in young women with breast cancer and long-term quality of life aspects have to be taken into account. The safe performance of sentinel lymph node biopsy in N+ patients with or without neoadjuvant therapy is the current focus of several large randomized controlled trials. In San Antonio, new data about the impact of micro-metastases or extracapsular extension were presented. The 10-year follow-up data from the AMAROS study demonstrated similar axillary recurrence rates in patients with positive sentinel nodes undergoing complete axillary dissection or radiotherapy only. The RAPID study showed that in patients with unifocal small DCIS (Ductal carcinoma in situ) or invasive cancer <3 cm, accelerated partial breast irradiation with three-dimensional conformal radiotherapy was not inferior compared to whole breast irradiation regarding ipsilateral recurrence rate, but the regimen has to be adapted to achieve similar cosmetic results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Breast-conserving surgery after neoadjuvant CHT
Quality of life of breast-conserving surgery vs. mastectomy
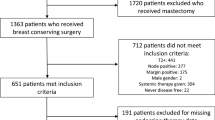
Despite effective neoadjuvant therapies and rising pCR rates, numbers of bilateral mastectomies have increased during the past years, also in patients without a hereditary breast cancer risk. Former studies have shown that although patients were eligible for BCS after neoadjuvant chemotherapy (nCHT), they underwent mastectomy (76% in Her2+ and 69% in triple-negative breast cancer, TNBC) [1, 2]. The Young Women’s Breast Cancer Study (YWS), a prospective multicenter cohort study, included 1302 breast cancer patients aged 40 or younger. Of these women, 315 patients received nCHT. While nCHT changed the percentage of patients eligible for BCS from 26 to 42%, it did not change the number of patients receiving BCS as a first surgical procedure: 44% received bilateral mastectomy, 33% unilateral mastectomy, and only 23% had BCS (Fig. 1). Among patients eligible for BCS receiving mastectomy, 35% had a pCR. Most common reasons for choosing mastectomy were patient preference (53%) or BRCA1, 2, or p53 mutation or strong family history (40%). 75% of patients who chose mastectomy underwent bilateral mastectomy.
These findings from the Dana Farber Cancer Center show that local treatment is not always based on clear medical indications and focused efforts to optimize surgical decision-making are needed.
Quality of life was evaluated in 560 patients of the YWS study, of whom 28% had BCS, and 89% of patients with uni- or bilateral mastectomy underwent reconstruction. A significant reduction in psychosocial well-being (68.4 vs. 75.9) and satisfaction with breasts and sexual well-being (49 vs. 57.4) was shown in patients who underwent uni- or bilateral mastectomy compared to BCS after a mean follow up of 5.8 years after surgery (Fig. 2). Knowledge of the potential long-term impact of surgery on quality of life is of critical importance for counseling young women about surgical decisions.
Sentinel lymph node
A retrospective study from the Memorial Sloan Kettering Cancer Center (MSKCC) examined microscopic extracapsular extension (mECE) in 685 patients with cT1-2 cN0 breast cancer and 1 or 2 positive sentinel nodes treated according to the ACOSOC Z0011 study. In 210 patients mECE was found, but there was no significant difference in 5‑year axillary recurrence with (2.3%) or without (1.3%) microscopic extracapsular extension (p = 0.84), so the authors conclude that this is no reason to perform ALND (Barrio A, et al. PD8-01).
The Ganea 2 study conducted in France proved the safety of performing sentinel node biopsy after neoadjuvant chemotherapy in initially node-negative patients, so ALND can safely be avoided [3].
In patients with initially positive axillary lymph nodes receiving neoadjuvant treatment, a number of studies are currently being conducted to investigate the safety of sentinel lymph node excision. The use of dual tracing and removal of >3 lymph nodes reduced the false-negative rate [4]. In a meta-analyses of 13 studies pooling 1921 cN+ patients, the false-negative rate was decreased to 4% by resecting >3 LNN at the sentinel biopsy [5]. After nCHT, ALND can be avoided in 48% of initially cN+ patients [6]. In 17% of patients with isolated tumor cells and 64% of patients with micrometastases in the sentinel after nCHT, additional positive axillary nodes were present. So isolated tumor cells and micrometastases still remain an indication for ALND, even when not detected on the intraoperative frozen section [7].
The value of preoperative imaging with MRI in neoadjuvantly treated patients was investigated in the ACRIN 6698 trial recently published in Radiology. This prospective multicenter trial conducted in the US examined the change in breast tumor apparent diffusion coefficient in MRI imaging to predict tumor response to nCHT. 272 patients with cT1-4 cN0 received 12 weekly doses of paclitaxel followed by 12 weeks of treatment with four cycles of anthracycline. In MRI, the longest diameter (LD) and the functional tumor volume (FTV) correlated well with tumor response (0.80–0.85), especially in hormone receptor-negative/Her2+ and TNBC patients. Thus, a change in breast tumor apparent diffusion coefficient at MRI predicted complete pathologic response to neoadjuvant chemotherapy [8].
AMAROS, radiotherapy
Emiel Rutgers from the Netherlands Cancer Institute in Amsterdam presented the 10-year follow-up data from the AMAROS study [9]. 1425 patients with T1-2 cN0 breast cancer undergoing BCT or mastectomy with positive sentinel lymph node were randomized to receive either complete axillary dissection (ALND) or radiotherapy including level 1–3 and medial supraclavicular (AxRT). Patients with >4 positive lymph nodes after ALND received additional AxRT.
The 10-year axillary recurrence rate was 0.93% in the ALND group and 1.82% in the RT group, which was not significant (Fig. 3).
There was no significant difference in disease-free, distant metastasis-free, or overall survival. Lymphedema of the arm was measured after 1, 3, and 5 years, and was significantly worse after ALND at all timepoints (29.4 vs. 14.6% after 5 years). Concerning shoulder function, there was a trend towards impaired shoulder movement after AxRT only in the first year. Therefore, both ALND and AxRT provided excellent regional control in patients with positive sentinel nodes undergoing breast-conserving therapy or mastectomy. As shown in many studies before, axillary recurrence was a very rare event. An interesting finding was the decreased incidence of lymphoedema of the arm after AxRT compared to surgery.
Timothy Whelan presented the prospective randomized RAPID study conducted in Canada comparing accelerated partial-breast irradiation (APBI) with three-dimensional conformal radiotherapy (3D-CRT) versus-whole breast irradiation (WBI) after breast cancer surgery in 2135 patients with unifocal ductal carcinoma in situ (DCIS) or invasive cancer <3 cm, N0 [10]. APBI is a non-invasive technique treating the surgical cavity plus 1 cm of surrounding tissue with 3–5 non-coplanar fields using 3D-CRT or IMRT at a dose of 38.5 Gy given in 10 fractions within 1 week or less. It was compared to standard WBI using standard tangential fields with 50 Gy (25 fractions) or 42.5 Gy (16 fractions), with or without a boost (10 Gy, 4–5 fractions) for moderate- to high-risk patients. ABPI was not inferior to WBI in terms of local recurrence. In-breast tumor recurrence was very low in both groups. Early toxicity was less with APBI, but there was increased late toxicity and worse cosmesis compared to WBI, especially with the twice-a-day regimen.
A large meta-analysis of 13,500 patients from 14 trials who underwent regional nodal irradiation was conducted by a collaborative group from Oxford (EBCTCG) [11], comparing patients participating in older trials (1961–1978) to those in newer trials (1989 onwards). Target coverage was better in the newer trials and heart dose was reduced to <8 Gy. Regional nodal irradiation had little effect on breast cancer mortality in the older trials (47.6 with regional nodal RT vs. 47.1% without), while overall mortality was high (66.9 vs. 64.1%). In the newer trials, breast cancer and overall mortality was significantly reduced; the absolute mortality reduction was greatest in N4+ patients (42.3% without LNN RT vs. 34.4% with LNN RT).
New data presented at the San Antonio Breast Cancer Symposium 2018 show that radiotherapy is an option for node-positive breast cancer patients and ALND is reserved for patients with a higher tumor burden in the axilla. In the era of neoadjuvant therapy with rising pCR, breast conservation should be aimed for whenever possible with regard to patients’ quality of life.
References
Golshan M, et al. Ann Surg. 2015;262(3):434–9.
Golshan M, et al. Breast Cancer Res Treat. 2016;160(2):297–304.
Classe J, et al. Breast Cancer Res Treat. 2019;173(2):343–52.
Boughey JC, et al. JAMA. 2013;310(14):1455–61.
Tee S, et al. Br J Surg. 2018;105:1541.
Mamtani A. Ann Surg Oncol. 2016;23(11):3467–74.
Moo T. Ann Surg Oncol. 2018;25(Suppl 3):685–6.
Partridge SC. Radiology. 2018;289(3):618–27.
Donker M. Lancet Oncol. 2014;15(12):1303–10.
Olivotto IA. J Clin Oncol. 2013;31(32):4038–45.
Clarke M. Lancet. 2005;366(9503):2087–106.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Exner declares that she has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Exner, R. Post San Antonio Breast Cancer Symposium 2018. memo 12, 257–260 (2019). https://doi.org/10.1007/s12254-019-0513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-019-0513-6