Summary
Although several large clinical trials have been conducted in order to investigate targeted inhibition of several molecular pathways in gastric cancer, only a limited number of targeted therapies have been introduced in clinical routine. Besides scientific interest, international guidelines recommend investigation of some distinct molecular alterations, which are associated with therapeutic consequences. These are (i) human epidermal growth factor receptor 2 (HER2), (ii) programmed death receptor 1 (PD-L1) and (iii) microsatellite instability (MSI). There are some emerging markers, such as Epstein–Barr virus (EBV), which might also be associated with a favorable response to immunotherapy. These routine and potential markers will be further discussed in the scope of this short review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
Testing for HER2 is an international consensus in the diagnostic work-up of metastatic gastrointestinal tumors. After the introduction of very promising results with immunotherapy, identifying emerging biomarkers for the prediction of treatment response has become of particular interest. In this regard, analysis of PD-L1 staining with CPS score and investigation of MSI show a strong correlation with treatment response; therefore, these investigations are already part of routine diagnostic biomarker investigations in the USA. Potential biomarkers for the prediction of successful treatment with immunotherapy such as EBV are promising; however, clinical and investigational data are not complete. There are several clinical trials, which test potential biomarkers for response prediction to immunotherapy such as tumor mutation burden, distinct labor alterations (neutrophil/lymphocyte ratio), or content of gut microbiome. These are investigational biomarkers and have no application for the routine assessments.
-
In case of resectable settings, molecular profiling and biomarker investigations are still in early phases, where no routine recommendation for the diagnostic procedures is available.
Introduction
Gastric cancer is a major contributor to global disease burden [1]. Even though survival has steadily increased during the past decades independent of the tumor stage, the prognosis remains poor [2]. Thus, molecular pathways, which drive tumor progression and metastasis, are of high clinical interest for the development of new targeted therapeutic approaches.
HER2
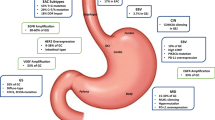
Human epidermal growth factor receptor 2 (HER2) is overexpressed in up to 20% of all gastroesophageal (GE) tumors [3]. There exists varying information on the expression of HER2 and its association with the prognosis of this malignant disease. Although HER2 positivity is mainly associated with poorer survival [4], comparable survival times with HER2-negative patients were also shown [4]. Recently, Gu et al. demonstrated a meta-analysis of the prognosis of HER2-positive patients, where equal survival rates between HER2-negative and -positive patients were observed [5].
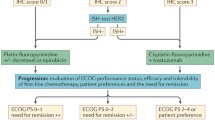
The pivotal ToGA (Trastuzumab for Gastric Cancer) trial was the first randomized, prospective, multicenter phase III trial to study the efficacy of first-line trastuzumab (a monoclonal antibody against HER2) in patients with HER2-positive advanced, metastasized or relapsed GE tumors [3]. Patients were randomly assigned to receive the standard chemotherapy combination of cisplatin plus fluorouracil/capecitabine with or without trastuzumab. Median overall survival (OS) was 13.8 months in the trastuzumab group, compared with 11.1 months in the control group (hazard ratio 0.74; 95% confidence interval [CI] 0.60–0.91; p = 0.0046). The longest survival (median 16 months) was seen in patients with the highest HER2 protein overexpression (defined by 3+ positive) and HER2 amplification. On the basis of this study, trastuzumab in combination with cisplatin and a fluoropyrimidine has been approved for the first-line treatment of advanced, metastasized or relapsed HER2-positive GE tumors. Consequently, an international consensus on the investigation of HER2 expression in advanced or metastatic settings was reached.
Two retrospective patient series investigating beyond progression trastuzumab continuation suggested a benefit in terms of an extension of overall survival and progression-free survival [6, 7]. However, two large clinical phase III trials investigating the anti-HER2 tyrosine kinase inhibitor lapatinib and chemotherapy/trastuzumab conjunction drug T‑DM1 in second-line treatment of HER2-positive patients, who received trastuzumab previously, failed to show a survival benefit in further lines [8, 9]. A possible reason for this failure was potentially the conversion of HER2 positivity to a negative state. A phase II trial from Japan evaluated trastuzumab treatment beyond progression and showed that HER2 positivity is lost in up to 70% of patients, which might lead to an anti-HER2 treatment inefficiency [10]. There is lack of evidence whether patients with maintained HER2 positivity in second-line settings would benefit from an anti-HER2 treatment.
HER2 inhibition in combination with neoadjuvant treatment is currently under investigation in three large clinical trials. The HER-FLOT trial is a phase II trial, where HER2-positive resectable GE tumor patients receive a combination of trastuzumab and the chemotherapy regimen FLOT (5-flurourocil, leucovorin, oxaliplatin, docetaxel) [11]. This trial published the interim results in ASCO 2014, where a pathological complete response rate of 22% could be achieved. In two further phase II trials, trastuzumab together with pertuzumab, another monoclonal antibody directed against HER2, and chemotherapy will be investigated in HER2-positive GE tumor patients [12, 13]. No data from these trials are available yet. Testing for HER2 expression in resectable settings might be useful for further treatment decision, when the patients relapse. However, therapeutic consequences in the neoadjuvant setting do not exist.
TCGA and molecular characterization
Recently, advances in technology and high-throughput analysis have improved our understanding of the genetic basis of gastric cancer. To provide a roadmap for patient stratification and trials of targeted therapies, the Cancer Genome Atlas (TCGA) Research Network has characterized 295 primary gastric adenocarcinomas and proposed a new classification of four different tumor subtypes consisting of Epstein–Barr virus positive, microsatellite instability (MSI), genomically stable and chromosomal instability subtypes [14].
PD-L1
Programmed cell death ligand 1 (PD-L1) is a 40-kDA transmembrane protein that is activated among many cancer types and leads to an immunosuppressive tumor microenvironment. Thus, inhibition of PD-L1 and its receptor PD‑1 have been intensively studied as novel treatment concepts in various cancer types [15]. A phase Ib clinical trial showed a promising overall response in gastric cancer when treated with the anti-PD‑1 antibody pembrolizumab [16]. A further phase II trial emphasized this response in PD-L1 combined positive score (CPS) ≥1% patients; thus, pembrolizumab was approved in the USA for this indication [17]. Furthermore, a recent phase III trial in heavily pretreated patients with gastric cancer demonstrated an efficacy with another PD‑1 inhibitor, nivolumab, in an Asian population, which led to its approval as salvage treatment in Japan [18]. Median OS was 5.26 months in the nivolumab group and 4.14 months in the placebo group (hazard ratio 0.63, 95% CI 0.51–0.78; p < 0.0001). Interestingly, pembrolizumab demonstrated different results in recent phase III trials in second line settings. In the Keynote-61 trial, pembrolizumab was not effective as second-line treatment option in PD-L1-positive preselected patients [19]. However, in the Keynote-181 trial, pembrolizumab was demonstrated to be effective in both adenocarcinoma and squamous cell carcinoma in second line, when CPS was ≥10% [20]. The Keynote-62 trial was presented within the ASCO 2019 [21]. The results of this study, which tested pembrolizumab as first-line treatment in advanced and metastasized gastroesophageal cancer, might indicate a survival benefit of patients with a CPS ≥10%. However, further investigation is necessary, since some subgroups developed a rapid progress despite having a CPS ≥10%.
According to National Comprehensive Cancer Network (NCCN) guidelines, CPS should be investigated in gastroesophageal carcinoma patients if metastatic disease is suspected. It is, however, important to mention that neither pembrolizumab nor nivolumab has treatment approval by European authorities; thus, no evident recommendation for CPS testing can be made. Nevertheless, in many large European centers CPS is investigated routinely in metastatic settings.
MSI
In gastric cancer, many studies have been conducted concerning the clinical and pathological characteristics of MSI. The majority of these studies show an association of a high MSI (MSI-H) with older age, female gender, distal third of the stomach, intestinal pathology, lower pTNM stage and lower number of infiltrated lymph nodes [22,23,24,25,26]. MSI‑H gastric cancer is generally characterized by some distinct genetic features including increased number of tumor infiltrating lymphocytes and PD-L1 positivity [22, 26]. It is surmised that around 20% of all western gastric tumor cases are MSI‑H [22, 26].
In 2017 immunotherapy with pembrolizumab was approved by the Food and Drug Administration (FDA) for the treatment of unresectable or metastatic, MSI‑H solid tumors that have progressed following prior treatment and which have no satisfactory alternative treatment options [27]. Based on this “tissue agnostic approval”, investigation of MSI in tissues of all tumor types might have a therapeutic consequence. However, it is again important to mention that this kind of treatment has not been approved by European authorities.
The British MAGIC trial demonstrated a survival benefit for patients with resectable cancer when treated perioperatively with the chemotherapy combination epirubicin, oxaliplatin, and capecitabine [28]. However, a recent post hoc investigation of the tissue samples of the MAGIC trial suggested that those patients with MSI‑H tumors benefited less from chemotherapy, indicating other treatment strategies should be offered for this selected patient group [29]. The post hoc investigation of the CLASSIC trial, where Asian patients were randomized to adjuvant chemotherapy versus surgery alone, did again show no survival benefit for patients with MSI‑H, when postoperative treatment is given [30]. Randomized prospective trials with MSI‑H patients, where chemotherapy in the resectable setting is omitted, are under investigation and will show us whether we could treat these patients solely with surgery or with an alternative therapy, such as immunotherapy. Therefore, it is too early now to say that MSI should be routinely tested in resectable settings.
EBV
Tumor Epstein–Barr virus (EBV) positivity is an emerging marker, which might be introduced in personalized treatment for gastric cancer. A positivity rate of 8–10% in gastroesophageal cancers is estimated [31]. Unlike other EBV-associated malignancies, the distribution of EBV-associated gastric carcinoma worldwide is approximately even. The prognostic value of EBV on the survival is not fully clarified; however, several studies indicate a better prognosis associated with EBV positivity [32,33,34]. Additional studies show an enhancement of PD-L1 expression in EBV-positive gastroesophageal tumors, which might suggest that immunotherapy targeting the PD-L1/PD‑1 axis might be of benefit in this subgroup [35, 36]. Recently, a phase II biomarker trial demonstrated a 100% overall response rate in gastroesophageal cancer patients with EBV positivity, when treated with pembrolizumab in a second-line setting [37]. Due to the lack of evidence in large clinical trials, investigation of EBV is not routinely recommended in international guidelines. Nevertheless, testing for EBV is already part of routine diagnostics in many large academic hospitals.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
SEER Cancer Stat Facts: Stomach Cancer. https://seer.cancer.gov/statfacts/html/stomach.html. Accessed 25 Sept 2019.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Van Cutsem E, Boni C, Tabernero J, Massuti B, Middleton G, Dane F et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol. 2015;26(1):149–56.
Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35(6):5315–21.
Al-Shamsi HO, Fahmawi Y, Dahbour I, Tabash A, Rogers JE, Mares JE et al. Continuation of trastuzumab beyond disease progression in HER2-positive metastatic gastric cancer: the MD Anderson experience. J Gastrointest Oncol. 2016;7(4):499–505.
Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–65.
Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Akitaka Makiyama KS, Junji Kawada, Tomomi Kashiwada, Ayumu Hosokawa, Yoshiki Horie, Hironaga Satake, et al. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT). ASCO. J Clin Oncol 2018;36:4011. https://doi.org/10.1200/JCO.2018.36.15_suppl.4011.
Hofheinz RSH‑B, Thuss-Patience PC, Kunzmann V, Fuchs M, GraevenNils Homann U, Heinemann V, et al. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the AIO Gastric Cancer Study Group. ASCO. J Clin Oncol 2014;32:4073. https://doi.org/10.1200/jco.2014.32.15_suppl.
Lordick F. Integration of Trastuzumab, with or without Pertuzumab, into Perioperative Chemotherapy of HER2-Positive Stomach Cancer: The INNOVATION Trial (EORTC-1203-GITCG). Oncol Res Treat. 2016;39(3):153–4. discussion 155.
Hofheinz RGH, Borchert K, Kretzschmar A, Ebert MP, Ettrich TJ, Koenigsmann M, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma: Petrarca—A phase II trial of the German AIO. ASCO. J Clin Oncol 2017;35. https://doi.org/10.1200/JCO.2017.35.15_suppl.TPS4133
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al. Safety, activity, and immune correlates of anti-PD‑1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–26.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e180013.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–33.
Takashi Kojima KM, Francois E, Hsu C‑H, Moriwaki T, Sung-Bae K, Se-Hoon L, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. ASCO GI. J Clin Oncol 2019;37:2. https://doi.org/10.1200/JCO.2019.37.4_suppl.2
Josep Tabernero EVC, Bang Y‑J, Fuchs CS, Wyrwicz L, Keun Wook L, Kudaba I, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. ASCO. J Clin Oncol 2019;37. https://doi.org/10.1200/JCO.2019.37.18_suppl.LBA4007
Polom K, Marano L, Marrelli D, De Luca R, Roviello G, Savelli V, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg. 2017. https://doi.org/10.1002/bjs.10663.
Kim Y, Cho MY, Kim J, Kim SN, Oh SC, Lee KA. Profiling cancer-associated genetic alterations and molecular classification of cancer in Korean gastric cancer patients. Oncotarget. 2017;8(41):69888–905.
Polom K, Marrelli D, Pascale V, Ferrara F, Voglino C, Marini M, et al. The pattern of lymph node metastases in microsatellite unstable gastric cancer. Eur J Surg Oncol. 2017;43(12):2341–8.
Kim KJ, Yang HK, Kim WH, Kang GH. Combined prognostic effect of PD-L1 expression and immunoscore in microsatellite-unstable advanced gastric cancers. Oncotarget. 2017;8(35):58887–902.
Cho J, Lee J, Bang H, Kim ST, Park SH, An JY et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017;8(8):13320–8.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD et al. PD‑1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki-Wilson S, Eltahir Z et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol. 2017;3(9):1197–203.
Choi YY, Kim H, Shin SJ, Kim HY, Lee J, Yang HK et al. Microsatellite Instability and Programmed Cell Death-Ligand 1 Expression in Stage II/III Gastric Cancer: Post Hoc Analysis of the CLASSIC Randomized Controlled study. Ann Surg. 2019;270(2):309–16.
Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M. Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol. 2008;14(27):4347–51.
Cho J, Kang MS, Kim KM. Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response. J Gastric Cancer. 2016;16(1):1–7.
van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22(4):664–70.
Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105(1):38–43.
Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, et al. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am J Surg Pathol. 2016;40(11):1496–506.
Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K et al. Comprehensive molecular characterization of clinical responses to PD‑1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.C. Puhr received travel support from Eli Lilly and Roche. A. Ilhan-Mutlu participated in advisory boards from Merck Sharp & Dohme and Servier, received lecture honoraria from Eli Lilly and Servier, is the local principle investigator for clinical trials sponsored by Bristol-Myers Squibb and Astellas.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Puhr, H.C., Ilhan-Mutlu, A. Molecular profiling in gastroesophageal cancer—clinical routine and future perspective. memo 13, 440–444 (2020). https://doi.org/10.1007/s12254-019-00534-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-019-00534-7