Abstract
The high frequency of prostate cancer in the population of the industrial nations and the lack of curative therapies for advanced hormone refractory disease mandates the investigation of novel, more efficient therapeutic approaches. Recent advances in rodent models have evidenced the therapeutic effects of antiprostate cancer vaccines, T cell transfer strategies and blockade of the negative regulator of T cell activation CTLA-4. Besides, several immunotherapeutic products have already been investigated in later-phase clinical trials and shown clinical benefit while maintaining an excellent quality of life for the treated patients, in particular, when compared with chemotherapy. However, there is also evidence for a synergistic effect of conventional treatment strategies and immunological antitumor approaches. For example, thermal ablation which is applied to destroy tumor tissue may help to activate tumor-specific T cells by elevating the presentation of tumor antigens to the immune system. Based on the experimental and clinical data the role of immune-based therapies is likely to become more prominent in the future years. This review summarizes results from contemporary immunologic therapies and clinical trials against prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Review article
Prostate cancer is the most common cancer found in men [1]. Effective treatment of metastatic prostate cancer currently involves radiation therapy, chemotherapy, and/or androgen ablation [2]. However, men with metastatic prostate cancer almost always progress after primary androgen ablation and develop castration-resistant prostate cancer after a median time of 18–24 months [3]. Other methods of hormonal manipulation such as estrogens or ketoconazole can improve the outcome in a subpopulation of patients with metastatic castration-resistant prostate cancer (mCRPC), but this benefit is usually short-lived [4]. A Canadian phase III randomized clinical trial (RCT) comparing mitoxantrone plus prednisone with prednisone alone established the palliative benefit of mitoxantrone in approximately 30 % of symptomatic men with mCRPC [5] and recently Docetaxel plus prednisolon was shown to have considerable effects in mCRPC [6].
Overall, for patients with hormone-refractory prostate carcinoma effective therapies are rare and prognosis is still very poor [7]. While chemotherapy has shown some success, it is relatively toxic in contrast to immunotherapeutic strategy.
Since the presence of lymphocytes within prostate tumor microenvironment [8] and circulating tumor-reactive T cells in the peripheral blood of prostate cancer patients [9] have been reported, it was suspected that this tumor entity elicits immune responses. The detailed analyses of the prostate tumor-infiltrating cells revealed CD8+ T cells [10], regulatory FoxP3+ cells (Treg) [11], and Th17 cells [12]. The presence alone of these cells does not necessarily proves their functional pro- or antiinflammatory function in the tumor microenvironment. However, dysfunctional T cells both systemically and at the tumor site [13] indicate that CTL may receive suppressive signals from other neighboring cells. The presence of Tregs at the tumor site can inhibit effector T cell trafficking and function. However, studies depleting Tregs have had mixed results in mouse models [14]. Thermal ablation to destroy tumor tissue may help activate tumor-specific T cells by elevating the presentation of tumor antigens to the immune system. Conversely, it was shown that Treg are recruited into tumor tissues via a CXCR4/CXCL12-dependent mechanism in which the chemoattractant was produced by antigen-presenting cells [15].
The vaccination with autologous monocyte-derived dendritic cells (DCs) pulsed with prostate-specific antigens has been shown to be effective in mice and men [16]. DCs pulsed with tumor lysates, peptide transfection of RNA or DC-tumor cell fusions have been shown to induce tumor-specific cytotoxic T-cell (CTL) response in vitro and antitumoral immune responses in animal models or human studies [17,18]. In the various prostate cancer vaccination strategies different peptides have been used. Ideally, the antigen is found at low levels in normal tissue and at high levels in the tumor tissue. Different antigens have been studied, and prostate-specific antigen (PSA) is found in prostate tissue and very low levels in normal breast and salivary gland tissue. The use of PSA peptides for vaccine development was further supported by the identification of several class I human leukocyte antigen A2 (HLA-A2)-restricted T-cell epitopes within the PSA-coding sequences [19] and HLA-A2 positive matured DCs pulsed with these epitopes had been shown to effectively activate PSA-specific CTL against PSA-expressing tumor cells [20,21]. Another antigen is prostate stem cell antigen (PSCA) which is also overexpressed in most prostate cancers. A phase I/II trial demonstrated that vaccination with PSA/PSCA peptide-loaded, autologous DCs induced cellular responses primarily in immunocompetent patients, which was associated with clinical benefit [22]. In this study, flow cytometry-based HLA tetramer analysis detected high frequencies of peptide-specific T cells after two vaccinations. The DTH immune response correlated with the overall survival of the patients treated with the vaccine.
Sipuleucel-T (APC8015), an immunotherapy product consisting of antigen-presenting cells, loaded with a recombinant fusion protein consisting of prostatic acid phosphatase linked to granulocyte-macrophage colony-stimulating factor, demonstrated in a phase III, placebo-controlled trial an improvement in the median time to progression [23,24]. The improvement in overall survival was 4.5 months for sipuleucel-T-treated patients compared with the placebo group [23,24].
Overall, the response rates to vaccination are low and a better understanding of factors that impact the microenvironment and the function of antigen-specific CTLs need further exploration. A recent study could show that the antitumoral CTL function following immunization is counteracted by the parallel expansion of Treg [25] which prevent effective tumor rejection. Therefore, the specific depletion of Treg was undertaken and improved immune responses [26].
Besides Treg residing in the tumor microenvironment, the role of androgen in modulating immune function, and the consequence of androgen removal/blockade on adaptive immune responses has been investigated in detail [25]. Androgen is generally regarded as immunosuppressive and hormone removal increases T cell function in autoimmune disease models [27,28]. Based on these observations it is conceivable that androgen removal may augment T cell responses following immunization against prostate tumor antigens, including self-proteins. Compatible with a role of androgen, a CD8+ T cell response to a prostate self-antigen was only elicited if the immunization preceded castration [29]. Conversely, the function of transgenic CD8+ T cells specific for PSA was enhanced until 4 weeks postcastration [30]. In a CD4+ transgenic T cell model, androgen deprivation and vaccination were synergistic and increased T cell proliferation early after castration [31]. These studies indicate that androgen ablation is capable to enhance antitumor immune responses to prostate-specific tumor antigens.
The antitumor activity of T cells in the microenvironment of prostate cancer may be restrained by their expression of the inhibitory T-cell coreceptor cytotoxic T-lymphocyte antigen 4 (CTLA-4) also known as CD152. A strategy to enhance antitumor immune responses is the use of ipilimumab, a blocking antibody against CTLA-4. In support of this concept, recent data indicate that a combination of cryoablation with CTLA-4 blockade enhances antitumor immunity against prostate cancer [32]. A recent clinical phase I dose-escalation trial including 28 patients combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic mCRPC [33]. A response with respect to the PSA level was observed in 25 % and the toxicity was limited [33]. A second clinical trial combined ipilimumab and a poxviral vaccine targeting prostate-specific antigen for patients with mCRPC and found that of the 24 patients who were chemotherapy naive, 58 % had PSA declines from baseline [34].
Based on the observation that lenalidomide can induce apoptosis in different malignant cell types by natural killer cell activation [35], that it can modulate the tumor cell microenvironment [36], block angiogenesis [37] and proliferation, it was also studied for its effect in prostate cancer [38]. In a clinical study on nonmetastatic biochemically relapsed prostate cancer, lenalidomide had acceptable toxicity and was associated with long-term disease stabilization [38].
Conclusion
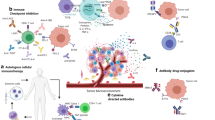
Results from the different studies on immunotherapy of prostate cancer imply that combination immunotherapy, with strategies to deplete Treg, transfer of tumor-specific T cells, and CTLA-4 blockade can lead to an increased proportion of functional T cells that can efficiently access the cancerous glands, which could be in the future effective at eliminating or preventing the development of castration-resistant prostate cancer (Fig. 1).
Ipilimumab, sipuleucel-T, and lenalidomide are novel therapeutic options targeting the prostate cancer microenvironment. By blocking cytotoxic T-lymphocyte antigen-4 (CTLA-4), ipilimumab neutralizes the negative regulator of T cell activation. The natural negative regulator that can be engaged by B7 molecules is CTLA-4 which then inhibits T cell activation. Sipuleucel-T consists of antigen-presenting cells, loaded with a recombinant fusion protein which is prostatic acid phosphatase linked to granulocyte-macrophage colony-stimulating factor. Lenalidomide can induce immunomodulation, modification of tumor cell microenvironment, and inhibition of angiogenesis
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Germany, ZE 872/1-1 (to R.Z.).
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300.
Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;5:389–96.
Eisenberger MA, Walsh PC. Early androgen deprivation for prostate cancer? N Engl J Med. 1999;341:1837–8.
Ryan CJ, Small EJ. Role of secondary hormonal therapy in the management of recurrent prostate cancer. Urology. 2003;62:87–94.
Tannock I, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64.
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
Silvestris N, Leone B, Numico G, et al. Present status and perspectives in the treatment of hormone-refractory prostate cancer. Oncology. 2005;69:273–82.
McArdle PA, Canna K, McMillan DC, et al. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91:541–3.
Elkord E, Rowbottom AW, Kynaston H, et al. Correlation between CD8+ T cells specific for prostate-specific antigen and level of disease in patients with prostate cancer. Clin Immunol. 2006;120:91–8.
Roden AC, Moser MT, Tri SD, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–108.
Miller AM, Lundberg K, Ozenci V, et al. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–405.
Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61.
Zheng X, Gao JX, Zhang H, et al. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J Immunol. 2002;169:4761–9.
Tien AH, Xu L, Helgason CD. Altered immunity accompanies disease progression in a mouse model of prostate dysplasia. Cancer Res. 2005;65:2947–55.
Dürr C, Pfeifer D, Claus R, et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer Res. 2010;70:10170–81.
Schellhammer PE, Hershberg RM. Immunotherapy with autologous antigen presenting cells for the treatment of androgen independent prostate cancer. World J Urol. 2003;23:47–9.
Michiels A, Tuyaerts S, Bonehill A, et al. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 2005;12:772–82.
Jarnjak-Jankovic S, Pettersen RD, Saeboe-Larssen S, et al. Preclinical evaluation of autologous dendritic cells transfected with mRNA or loaded with apoptotic cells for immunotherapy of high-risk neuroblastoma. Cancer Gene Ther. 2005;12:699–707.
Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigen peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol. 1998;114:166–72.
Alexander RB, Brady F, Leffell MS. Specific T cell recognition of peptides derived from prostate-specific antigen in patients with prostate cancer. Urology. 1998;51:150–7.
Correale P, Walmsley K, Nieroda C, et al. In vitro generation of human cytotoxic Tlymphocytes specific for peptides derived from prostata-specific antigen. JNCI. 1997;89:293–300.
Thomas-Kaskel AK, Zeiser R, Jochim R, et al. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119:2428–34.
Paller CJ, Antonarakis ES. Sipuleucel-T for the treatment of metastatic prostate cancer: promise and challenges. Hum Vaccin Immunother. 2012;8:1–8.
Sims RB. Sipuleucel-T: autologous cellular immunotherapy for men with asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. J Cancer. 2011;2:357–9.
Tang S, Moore ML, Grayson JM, et al. Increased CD8+ T cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012;72:1975–85.
Akins EJ, Moore ML, Tang S, et al. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010;70:3473–82.
Fijak M, Schneider E, Klug J, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–72.
Radojevic K, Arsenovic-Ranin N, Kosec D, et al. Neonatal castration affects in trathymic kinetics of T cell differentiation and the spleen T-cell level. J Endocrinol. 2007;192:669–82.
Koh YT, Gray A, Higgins SA, et al. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–84.
Arredouani MS, Tseng-Rogenski SS, Hollenbeck BK, et al. Androgen ablation augments human HLA2.1-restricted T cell responses to PSA self-antigen in transgenic mice. Prostate. 2010;70:1002–11.
Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49.
Waitz R, Solomon SB, Petre EN, Trumble AE, Fassò M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res. 2012;72:430–9.
van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17.
Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8.
Zhu D, Corral LG, Fleming YW, et al. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–59.
Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163:380–6.
Lentzsch S, LeBlanc R, Podar K, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–4.
Keizman D, Zahurak M, Sinibaldi V, et al. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–76.
Conflict of interest
The author declares that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeiser, R. Immunotherapeutic approaches targeting prostate cancer and its microenvironment. memo 5, 94–97 (2012). https://doi.org/10.1007/s12254-012-0021-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-012-0021-4