Abstract
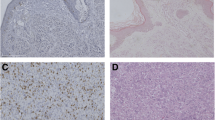
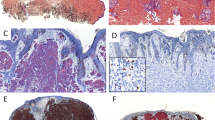
Vertical tumor thickness has great influence in the prognosis and staging of melanoma. The aim of this study was determination of the differences between melanoma tumor thickness in conventional hematoxylin and eosin (H&E) and immunohistochemical techniques. Thirty-six biopsy specimens were included in our study. For each sample, four adjacent tissue sections were stained with H&E, in addition S-100, Melan- A and HMB-45 staining was performed on the next serial sections. The mean thickness of tumor invasion was 2.16, 2.38, 2.22 and 2.29 mm in H&E, S-100, HMB45 and Melan-A sections evaluation, respectively. The mean difference of the Breslow thickness between H&E and S-100 and also, between H&E and Melan-A stained slides were statistically significant (p˂0.05) while no difference was found in the tumor thickness of the H&E and HMB45 staining evaluation (p = 0.278). Greater tumor thickness was observed in 25 lesions (69.4%) with S-100, 20 lesions (55.5%) with Melan-A and 17 (47.2%) lesions in HMB-45 rather than H&E staining. Conclusively, it appears that H&E staining cannot prove the actual size of melanoma invasion in some cases and immunohistochemical examination can be a complementary method in this situations. Of the melanoma associated immunomarkers, the combination of S-100 and Melan-A staining may suffice to measure depth of tumor invasion.


Similar content being viewed by others
References
Breslow A (1980) Prognosis in cutaneous melanoma: tumor thickness as a guide to treatment. Pathol Annu 15:1–22
Shaikh WR, Dusza SW, Weinstock MA et al (2015) Melanoma thickness and survival trends in the United States, 1989–2009. J Natl Cancer Inst 12:108(1)
Scolyer RA, Shaw HM, Thompson JF, Li LXL, Colman MH, Lo SK, McCarthy SW, Palmer AA, Nicoll KD, Dutta B, Slobedman E, Watson GF, Stretch JR (2003) Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol 27:1571–1576
Haydu LE, Stollman JT, Scolyer RA, Spillane AJ, Quinn MJ, Saw RPM, Shannon KF, Stretch JR, Bonenkamp JJ, Thompson JF (2016) Minimum safe pathologic excision margins for primary cutaneous melanomas (1–2 mm in thickness): analysis of 2131 patients treated at a single center. Ann Surg Oncol 23:1071–1081
Hurt MA, Santa Cruz DJ (1994) Malignant melanoma microstaging. History, premises, methods, problems, and recommendations—a call for standardization. Pathol Annu 29:51–74
Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW (2008) Immunohistochemical characteristics of melanoma. J Cutan Pathol 35:433–444
Plaza JA, Suster D, Perez-Montiel D (2007) Expression of immunohistochemical markers in primary and metastatic malignant melanoma: a comparative study in 70 patients using a tissue microarray technique. Appl Immunohistochem Mol Morphol 15:421–425
Paradela S, Fonseca E, Pita S, Kantrow SM, Goncharuk VN, Diwan H, Prieto VG (2009) Spitzoid melanoma in children: clinicopathological study and application of immunohistochemistry as an adjunct diagnostic tool. J Cutan Pathol 36:740–752
Buzaid AC, Gershenwald JE, Atkins MB, Ross ME (2016) Tumor node metastasis (TNM) staging system and other prognostic factors in cutaneous melanoma. UpToDate. UpToDate, Waltham
Drabeni M, Lopez-Vilaró L, Barranco C, Trevisan G, Gallardo F, Pujol RM (2013) Differences in tumor thickness between hematoxylin and eosin and Melan-A immunohistochemically stained primary cutaneous melanomas. Am J Dermatopathol 35:56–63
Xia J, Wang Y, Li F, Wang J, Mu Y, Mei X, Li X, Zhu W, Jin X, Yu K (2016) Expression of microphthalmia transcription factor, S100 protein, and HMB-45 in malignant melanoma and pigmented nevi. Biomed Rep 5:327–331
Orchard GE (1998) Melan A (MART-1): a new monoclonal antibody for malignant melanoma diagnosis. Br J Biomed Sci 55:8–9
Morton DL, Davtyan DG, Wanek LA, Foshag LJ, Cochran AJ (1993) Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 71:3737–3743
Urist MM, Balch CM, Soong SJ et al (1984) Head and neck melanoma in 534 clinical Stage I patients. A prognostic factors analysis and results of surgical treatment. Ann Surg 200:769–775
Dyson SW, Bass J, Pomeranz J, Jaworsky C, Sigel J, Somach S (2005) Impact of thorough block sampling in the histologic evaluation of melanomas. Arch Dermatol 141:734–736
de Vries TJ, Smeets M, de Graaf R, Hou-Jensen K, Bröcker EB, Renard N, Eggermont AMM, van Muijen GNP, Ruiter DJ (2001) Expression of gp100, MART-1, tyrosinase, and S100 in paraffin-embedded primary melanomas and locoregional lymph node, and visceral metastases: implications for diagnosis and immunotherapy. A study conducted by the EORTC melanoma cooperative group. J Pathol 193:13–20
Kim RH, Meehan SA (2017) Immunostain use in the diagnosis of melanomas referred to a tertiary medical center: a 15-year retrospective review (2001–2015). J Cutan Pathol 44:221–227
Penneys NS (1987) Microinvasive lentigo maligna melanoma. J Am Acad Dermatol 17:675–680
Flügge G, Rassner G (1989) Demonstration of S 100 protein in malignant melanoma of the skin. Pattern of distribution and significance for determination of tumor thickness. Hautarzt. 40:290–295
Prade M, Sancho-Garnier H, Cesarini JP, Cochran A (1980) Difficulties encountered in the application of Clark classification and the Breslow thickness measurement in cutaneous malignant melanoma. Int J Cancer 26:159–163
Prieto VG, Shea CR (2008) Use of immunohistochemistry in melanocytic lesions. J Cutan Pathol 35:1–10
Megahed M, Schön M, Selimovic D, Schön MP (2002) Reliability of diagnosis of melanoma in situ. Lancet. 359:1921–1922
Thum C, Hollowood K, Birch J, Goodlad JR, Brenn T (2011) Aberrant Melan-a expression in atypical fibroxanthoma and undifferentiated pleomorphic sarcoma of the skin. J Cutan Pathol 38:954–960
Bax MJ, Johnson TM, Harms PW, Schwartz JL, Zhao L, Fullen DR, Chan MP (2016) Detection of occult invasion in melanoma in situ. JAMA Dermatol 152:1201–1208
Kim J, Taube J, McCalmont TH, Glusac EJ (2011) Quantitative comparison of MiTF, Melan-a, HMB-45 and Mel-5 in solar lentigines and melanoma in situ. J Cutan Pathol 38:775–779
Helm TN, Helm KF (2014) Breslow thickness determined with the use of Immunohistochemical techniques could provide misleading information when used with prognostic models based on data obtained by conventional means. Am J Dermatopathol 36:757
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamyab-Hesary, K., Ghanadan, A., Balighi, K. et al. Immunohistochemical Staining in the Assessment of Melanoma Tumor Thickness. Pathol. Oncol. Res. 26, 885–891 (2020). https://doi.org/10.1007/s12253-019-00635-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00635-y