Abstract
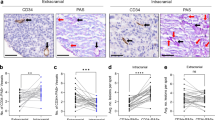
Brain metastasis is a frequent complication of the progression of malignant melanoma. In a previous study aquaporin 1 (AQP1) protein expression was found to be associated with increased mortality and decreased progression free survival in cutaneous melanoma. To explore further the potential of this marker we studied the AQP1 protein expression in 67 metastatic melanoma patients using immunohistochemistry. Primary tumor samples were acquired from patients with brain (BR) (n = 44) and extra-cranial (EC) (n = 23) metastases, while brain metastatic samples were collected during neurosurgical resection (n = 5). Patients with brain metastases had shorter overall survival (p = 0.02) and significantly higher AQP1 expression in the primary tumors (median H-score = 250 vs. 140, p = 0.044) as compared to patients of the EC metastasis group. AQP1 expression was found to be significantly lower in the brain metastases compared to the corresponding primary tumors (median H-score = 35 vs. 300 p = 0.01). However, in brain metastases AQP1 expression was heterogenous, AQP1 protein was more abundant in the melanoma cells far away from the capillaries as compared to tumor cells adjacent to vessels indicating a hypoxia-driven expression of AQP1. We suggest that AQP1 expression could well be a prognostic marker of brain metastatic potential of human cutaneous melanoma.




Similar content being viewed by others
References
Franceschini D, Franzese C, Navarria P, Ascolese AM, De Rose F, Del Vecchio M et al (2016) Radiotherapy and immunotherapy: can this combination change the prognosis of patients with melanoma brain metastases? Cancer Treat Rev 50:1–8
Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P (2016) Survival of patients with metastatic melanoma and brain metastases in the era of map-kinase inhibitors and immunologic checkpoint blockade antibodies: a systematic review. Cancer Treat Rev 45:38–45
Staudt M, Lasithiotakis K, Leiter U, Meier F, Eigentler T, Bamberg M, Tatagiba M, Brossart P, Garbe C (2010) Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer 102:1213–1218
Weidle UH, Birzele F, Kollmorgen G, Ruger R (2016) Dissection of the process of brain metastasis reveals targets and mechanisms for molecular-based intervention. Cancer Genomics Proteomics 13:245–258
Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, Kim SM, Park YY, Cheong JH, Woo HG, Mills GB, Fidler IJ, Lee JS (2011) Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. PNAS 108:17456–17461
Nygaard V, Prasmickaite L, Vasiliauskaite K, Clancy T, Hovig E (2014) Melanoma brain colonization involves the emergence of a brain-adaptive phenotype. Oncoscience 1:82–94
Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA (2013) Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci 14:1383–1411
Dome B, Paku S, Somlai B, Timar J (2002) Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J Pathol 197:355–362
Kusters B, Leenders WP, Wesseling P, Smits D, Verrijp K, Ruiter DJ et al (2002) Vascular endothelial growth factor-a(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res 62:341–345
Nicchia GP, Stigliano C, Sparaneo A, Rossi A, Frigeri A, Svelto M (2013) Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J Mol Med 91:613–623
Hu J, Verkman AS (2006) Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J 20:1892–1894
Timar JPL, Raso E (2007) Identification of the metastasis gene signature of human melanoma: dissection of tumor- and host components. Clin Exp Metastasis 24:211–316
Jaeger J, Koczan D, Thiesen HJ, Ibrahim SM, Gross G, Spang R, Kunz M (2007) Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser-microdissected melanoma tissues. Clin Cancer Res 13:806–815
Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J (2008) The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genet 1:13
Imredi E, Toth B, Doma V, Barbai T, Raso E, Kenessey I et al (2016) Aquaporin 1 protein expression is associated with braf v600 mutation and adverse prognosis in cutaneous melanoma. Melanoma Res 26:254–260
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur G, Haydu LE, Eggermont AMM, Flaherty KT, Balch CM, Thompson JF, for members of the American Joint Committee on Cancer Melanoma Expert Panel and the International Melanoma Database and Discovery Platform (2017) Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492
Fidler IJ, Schackert G, Zhang RD, Radinsky R, Fujimaki T (1999) The biology of melanoma brain metastasis. Cancer Metastasis Rev 18:387–400
Paku S, Dome B, Toth R, Timar J (2000) Organ-specificity of the extravasation process: an ultrastructural study. Clinical Exp Met 18:481–492
Jilaveanu LB, Parisi F, Barr ML, Zito CR, Cruz-Munoz W, Kerbel RS, Rimm DL, Bosenberg MW, Halaban R, Kluger Y, Kluger HM (2015) Plekha5 as a biomarker and potential mediator of melanoma brain metastasis. Clin Cancer Res 21:2138–2147
Gaziel-Sovran A, Osman I, Hernando E (2013) In vivo modeling and molecular characterization: a path toward targeted therapy of melanoma brain metastasis. Front Oncol 3:127
Barbai T, Fejos Z, Puskas LG, Timar J, Raso E (2015) The importance of microenvironment: the role of ccl8 in metastasis formation of melanoma. Oncotarget 6:29111–29128
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128:2527–2535
Kenessey I, Banki B, Mark A, Varga N, Tovari J, Ladanyi A et al (2012) Revisiting cb1 receptor as drug target in human melanoma. Pathol Oncol Res 18:857–866
Hasko J, Fazakas C, Molnar J, Nyul-Toth A, Herman H, Hermenean A et al (2014) Cb2 receptor activation inhibits melanoma cell transmigration through the blood-brain barrier. Int J Mol Sci 15:8063–8074
Chen G, Chakravarti N, Aardalen K, Lazar AJ, Tetzlaff MT, Wubbenhorst B, Kim SB, Kopetz S, Ledoux AA, Gopal YNV, Pereira CG, Deng W, Lee JS, Nathanson KL, Aldape KD, Prieto VG, Stuart D, Davies MA (2014) Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the pi3k pathway as a therapeutic target. Clin Cancer Res 20:5537–5546
Rakosy Z, Vizkeleti L, Ecsedi S, Voko Z, Begany A, Barok M et al (2007) EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int J Cancer 121:1729–1737
Abreu-Rodriguez I, Sanchez Silva R, Martins AP, Soveral G, Toledo-Aral JJ, Lopez-Barneo J et al (2011) Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of hif-1alpha. PLoS One 6:e28385
Simone L, Gargano CD, Pisani F, Cibelli A, Mola MG, Frigeri A, Svelto M, Nicchia GP (2018) Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma. J Cell Mol Med 22:904–912
Acknowledgements
We also thank Violetta Piurkó for her valuable contribution in immunohistochemistry. We are grateful for all the patients, their families and the clinicians who participated in this study, and consented to provide tissue for research.
Funding
Support was provided by Hungarian Scientific Research Fund Research Grant K-112371, the NAP_B13-2014- and 2017-1.2.1.NKP-0002 programs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
This manuscript has not been published previously. This study is not part of another larger clinical study. We state that no data have been fabricated or manipulated. We also state that the Result section contains our own data. All authors expressed their consent to publish this manuscript in the final form. Authors whose names appear on the submission have contributed sufficiently to this work and therefore share collective responsibility and accountability for the demonstrated results.
Rights and permissions
About this article
Cite this article
Imrédi, E., Liszkay, G., Kenessey, I. et al. Aquaporin-1 Protein Expression of the Primary Tumor May Predict Cerebral Progression of Cutaneous Melanoma. Pathol. Oncol. Res. 26, 405–410 (2020). https://doi.org/10.1007/s12253-018-0513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0513-6