Abstract
The combination of cisplatin and gemcitabine is still one of the most frequently used first-line chemotherapy scheme in patients with advanced non-small cell lung cancer (NSCLC), in which tyrosine kinase inhibitors (TKIs) cannot be administered. Unfortunately, more than half of the patients have no benefit from chemotherapy but are still exposed to its toxic effects. Therefore, single nucleotide polymorphisms (SNPs) in the genes involved in nucleotide excision repair (NER) mechanism may be a potential predictive factor of efficiency of cytostatic based chemotherapy. The aim of the study was to evaluate the correlation between SNPs of the genes involved in NER mechanism and the effectiveness of chemotherapy based on cisplatin and gemcitabine in patients with advanced NSCLC. The study group included 91 NSCLC patients treated with first-line chemotherapy using cisplatin and gemcitabine. Genotyping was carried out using a mini-sequencing technique (SNaPshot™ PCR). The median progression-free survival (PFS) was significantly shorter in carriers of CC genotype of the XPD/ERCC2 (2251A > C) gene compared to patients with AA/AC genotypes (2 vs. 4.5 months; p = 0.0444; HR = 3.19, 95%CI:1.03–9.91). Rare CC genotype of XPD/ERCC2 gene, may be considered as an unfavorable predictive factor for chemotherapy based on cisplatin and gemcitabine in patients with advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is a leading cause of cancer-related deaths in the developed countries. In the world, more than 1.5 million new cases and deaths due to lung cancer are reported every year. Non-small cell lung cancer (NSCLC) is the most frequent histopathological type of lung cancer and it is diagnosed in about 85% patients. Usually NSCLC is diagnosed in locally advanced or advanced stage of progression (IIIA – inoperable cases, IIIB, IV), which disqualifies these patients from radical surgery. Standard chemotherapy, often combined with radiotherapy, remains the main method of treatment. Standard first-line chemotherapy in patients with advanced NSCLC is based on combination of platinum compounds (mainly cisplatin) with a second generation drug (e.g. gemcitabine). However, most cytostatic-based treatment regimens used in NSCLC are characterized with similarly poor effectiveness. Objective response rate (ORR) is obtained usually in less than 40% of cases, median of overall survival (OS) increases only up to 1.5 months in comparison with best supportive care. On the other hand, it is usually associated with high systemic toxicity (nephro-, hepato- and haematological toxicity) [1,2,3].
More benefit for lung cancer patients is provided by molecularly targeted therapy (e.g. erlotinib, afatinib, crizotinib). Despite the fact, that we already have several generations of targeted drugs, this type of treatment can be used only in selected patients with specific genetic abnormalities: activating mutations in EGFR (9–51% depends by race) or ALK rearrangements (3–7%). A new promising tool for lung cancer therapy is the immune checkpoint inhibition, especially focused on programmed death-1 (PD-1)/programmed death ligand −1 (PD-L1) signalization [4,5,6]. Nevertheless, despite undeniable breakthrough related with the introduction of new molecularly targeted drugs, a substantial number of patients still receive cytostatics as a part of multidisciplinary treatment. Due to this fact, this type of therapy is addressed predominantly to patients diagnosed with adenocarcinoma, in whom the above mentioned rare molecular disorders occur more frequently [4, 5]. Therefore, it appears to be an interesting alternative to investigate new therapeutic targets and agents to maximize the benefits from the “old”, well known, cytostatics.
Platinum compounds react with DNA to form intra- as well as inter-strand cross-links. Then, the formed abnormal DNA structure (the so-called DNA adducts) may cause DNA strand breaks, inhibition of transcription and alterations of proteins encoding, which, in most cases, lead to apoptosis. Regarding gemcitabine mechanism of action, it is based on its incorporation into nucleic acids, which subsequently leads to inhibition of DNA replication and may also induce apoptosis [7].
Over thirty specialized proteins (including: ERCC1, XPA, XPC, XPD and XPG) are involved at various stages of NER mechanism, which is one of the major mechanism of DNA repair systems. Therefore, any change regarding their expression level and functioning (e.g. single nucleotide polymorphisms - SNPs) may lead to alteration of effectiveness of cytostatics (if their mechanism of action is based on direct or indirect DNA damage) [8].
Studies recorded over the past few years demonstrate that, in some subgroups of NSCLC patients receiving standard chemotherapy, genetic predisposition (e.g. SNPs), especially in genes encoding DNA repair proteins, may have the potential to become predictive or prognostic factors [9,10,11,12]. However, we still do not have any conclusive results and also their role as predictive or prognostic factors has not been definitively clarified.
The aim of this study was the assessment of the relationship between 8 SNPs of 5 genes involved in NER mechanism (ERCC1, XPA, XPC, XPD and XPG) and the effectiveness of cisplatin and gemcitabine based chemotherapy in patients with advanced NSCLC.
Materials and Methods
Study Group
Our study group (n = 91) included 67% male and 33% female Caucasian patients with NSCLC, recruited in 2010–2013 at the Department of Pneumonology, Oncology and Allergology, Medical University of Lublin. Each patient’s detailed demographic and clinical data were collected. The stage of disease was determined based on the TNM classification (VII edition by UICC): 30.8 and 69.2% of patients were in stage IIIB and IV, respectively. The basic inclusion criterion was first-line chemotherapy treatment based on platinum compounds and gemcitabine (median cycles of chemotherapy was 4). Detailed data of patients are specified in Table 1. The study protocol was approved by the Committee of Ethics and Research at the Medical University of Lublin (approval no.: KE-0254/142/2010). The informed consent was obtained from all individual participants enrolled in the study.
Methods
Approximately 5 ml of peripheral blood was collected from each patient. After isolation of DNA (QIAamp DNA Blood Mini Kit, Qiagen, Canada) using a spectrophotometer (BioPhotometer plus cuvette equipped with UV / VIS filters, Eppendorf, Germany), purity and quantity of the isolated nucleic acids were evaluated. The next step was the use of mini-sequencing technique for genotyping (ABI PRISM® SnaPshot® Multiplex Kit, Life Technologies). An example of the genotyping result is shown in Fig. 1.
Genotyping results (capillary electrophoresis of SNaPshot® PCR products) From left: CC homozygote (8092C > A), AG heterozygote (934G > A), AA (19007C > T), TT (1385C > T) and GG (2704C > A) homozygotes, AC heterozygote (2251A > C), AG heterozygote (−4A > G), GG homozygote (3310C > G) (in certain cases analysis performed on the opposite strand where A = T and G = C)
Response to therapy was evaluated according to the RECIST (Response Evaluation Criteria in Solid Tumors) criteria (v.1.1). Common Toxicity Criteria (CTC) guideline (version no. 4.03) was used for toxicity assessment. Response to therapy, progression-free survival (PFS) and overall survival (OS) were correlated with demographic, clinical and genetic factors.
Statistical Analysis
Statistical analysis was performed using MedCalc 10 (MedCalc Software, Belgium). Results of p < 0.05 were considered as statistically significant. Hardy-Weinberg Equilibrium Eq. (HW) and the correlation between selected clinical and demographic factors and SNPs were assessed by Chi Square (χ2) test. Using the Kaplan-Meier method, the probability of PFS and OS depending on clinical factors, demographic factors and SNP variants was evaluated. The factors potentially affecting survival were evaluated using Cox regression model with a stepwise selection and minimum AIC factor (Akaike Information Criterion).
Results
The distribution of demographic and clinical characteristics (age, gender, smoking status, histology, PS and stage of disease) was independent of SNPs variants. The characteristics of the studied SNPs was shown in Table 2. The genotypes of all the examined genes were in Hardy-Weinberg equilibrium, except for the 2251A > C SNP of XPD/ERCC2 gene (p = 0.0019, χ2 = 9.679).
Response to Treatment
In the study population, there was no case of complete remission (CR). Progression of the disease (PD) was recorded in 45.1% of patients, whereas stable disease (SD) and partial response (PR) were observed in 17.6 and 37.3% of patients, respectively (therefore, control of the disease was achieved in 54.9%). For patients with worse performance status (PS), a higher risk of disease progression was reported (PS ≥ 1; OR = 4.9, 95%CI: 1.00–23.69; p = 0.0495). Also, when squamous cell carcinoma (OR = 3.71, 95%CI: 1.07–12.89; p = 0.0392) or the presence of anemia before starting chemotherapy (OR = 3.03, 95%CI: 1.20–7.64; p = 0.0189) was diagnosed a significantly higher risk of early progression was observed. As regards other demographic and clinical factors, there was no statistically significant difference in the response to treatment when first-line chemotherapy was used (Table 3). Moreover, in the case of all studied SNPs, non-significant effect on response to treatment was recorded (Table 4).
Progression-Free Survival
Median PFS for the study population was 4 months. Clinical factors associated with a shortened PFS in the study group were as follows: poor PS (2 vs 6 months; HR = 3.03, 95%CI: 1.61–5.88; p = 0.0006); anemia before chemotherapy (3 vs 6.5 months.; HR = 1.84, 95%CI: 1.12–3.02; p = 0.0154); stage IV of the disease (3 vs 7 months; HR = 1.92, 95%CI: 1.18–3.12; p = 0.0094), diagnosis of non-adenocarcinoma (3 vs 6 months; HR = 1.64, 95%CI: 1.01–2.68; p = 0,0456) or squamous cell carcinoma (2 vs 4.5 months; HR = 2.94, 95%CI: 1.24–6.96; p = 0.0140). Other evaluated clinical factors had no significant influence on PFS.
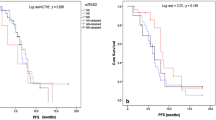
In patients with the CC genotype (2251A > C) of the XPD/ERCC2 gene, a significant decrease in median PFS, compared to patients with other polymorphic variants of this gene, was observed (2 vs 4.5 months; HR = 3.19, 95% CI: 1.03–9.91; p = 0.0444; Fig. 2). Statistically significant correlation between the duration of the PFS and the occurrence of individual genotypes was not demonstrated for the remaining studied SNPs. Detailed results regarding the impact of the demographic, clinical and genetic factors on PFS were shown in Tables 3 and 4.
With the use of Cox multivariate logistic regression, we have demonstrated that factors which significantly shortened PFS in patients treated with cisplatin/gemcitabine based chemotherapy (overall fit of the model; χ2 = 46.59; p = 0.0025) were the following: poor PS (PS = 2, HR = 5.78, 95%CI: 2.18–15.34; p = 0.0005), higher stage of progression (IV, HR = 3.21, 95%CI: 1.41–7.28; p = 0.0055), diagnosis of non-adenocarcinoma type of NSCLC (HR = 3.14, 95%CI: 1.14–8.61; p = 0.0270) and CC genotype (2251A > C) of XPD/ERCC2 gene (HR = 12.62, 95%CI: 1.23–129.43; p = 0.0337).
Overall Survival
Median OS for the study population was 12 months. Clinical factors associated with a shortened OS in the study group were: weight loss before the beginning of chemotherapy (7.5 vs 18 months; HR = 2.16, 95%CI: 1.04–4.46; p = 0.0376) and lack of subsequent lines of treatment (8 vs 16.5 months; HR = 2, 95%CI: 1.07–3.73; p = 0.0305). Poor PS shows a trend of only borderline significance (11 vs 21 months; HR = 1.95, 95%CI: 0.95–4.02; p = 0.07). Univariate analysis demonstrated that there was no significant correlation with the other studied clinical factors as well as SNPs and the length of OS. The influence of the polymorphism of XPD/ERCC2 on OS was shown in Fig. 3.
With the use of Cox multivariate logistic regression, we have demonstrated that factors which significantly shortened OS in patients treated with cisplatin/gemcitabine based chemotherapy (overall fit of the model; χ2 = 49.98; p = 0.0006) were the following: poor PS (PS = 2, HR = 7.31, 95%CI: 2.17–24.61; p = 0.0014), higher stage of progression (IV, HR = 3.96, 95%CI: 1.15–13.55; p = 0.0294), diagnosis of squamous cell type of NSCLC (HR = 6.79, 95%CI: 1.80–25.60; p = 0.0049), age below 70 years (HR = 7.39, 95%CI: 1.36–40,25; p = 0.0214), anemia before the beginning of chemotherapy (HR = 6.07, 95%CI: 1.36–27.09, p = 0.0188), lack of subsequent lines of chemotherapy (HR = 18.46, 95%CI: 4.31–78.95; p < 0.0001) and CC genotype (2251A > C) of XPD/ERCC2 gene (HR = 51.99, 95%CI: 1.19–2274.11; p = 0.0414).
Detailed data of response to treatment, PFS and OS were shown in Tables 3 and 4.
Discussion
The role of DNA repair proteins (especially those participating in NER mechanism) in the removal of the cisplatin adducts and gemcitabine induced alterations (although to a lesser extent) is well known [7,8,9]. Occurrence of SNPs in non-coding (3’UTR, promoter regions) or coding sequences of genes may lead to numerous alterations including changes in the structure, stability, folding, expression and function of proteins [13]. Therefore, testing of SNPs has a high potential to be applied in routine clinical practice. Thus, a number of studies (unfortunately mainly retrospective) were aimed at assessing the correlation between various SNPs of genes involved in DNA repair and the effectiveness of different treatment regimens in patients with advanced NSCLC [10,11,12, 14, 15]. In the available literature. The most frequently evaluated SNPs of XPD/ERCC2 gene, which can potentially be related to the effectiveness of chemotherapy based on platinum compounds and gemcitabine, were: 2251A > C and 934G > A [16,17,18,19,20]. A meta-analysis performed by Qin et al. on the basis of 24 studies (4468 patients with NSCLC) assessed the impact of both of the above described SNPs on treatment response, PFS, and OS in patients treated with first-line chemotherapy based on platinum compounds and next generation drug [21]. Sixteen studies considered objective response to first-line chemotherapy. They demonstrated that the SNP 2251A > C was not significantly correlated with the objective response to treatment. However, the results of an additional subgroup analysis showed a significant difference in response to treatment depending on the patients’ race. In the case of Asians (8 studies, 1795 patients) no significant association between genotype variant and the possibility of obtaining the response to treatment was noted, while in Caucasians (8 studies, 853 patients) beneficial effect of AA genotype was observed (OR = 1.35, 95% CI: 1–1,83, p = 0.05) [21]. Authors did not find significant differences in the length of PFS in carriers of various genotypes of XPD/ERCC2 gene (SNP 2251A > C). Subgroup analysis showed a significant increase in the risk of early progression in C allele carriers (CC or CA) compared to patients with AA genotype in Asian patients (HR = 1.39, 95% CI: 1.07–1.81, p = 0.015). However, no such correlation was found in Caucasians [21]. Also no significant differences in the length of OS depending on the occurrence of SNP 2251A > C of XPD/ERCC2 gene were recorded. Similarly, subgroup analysis showed no significant increase in the risk of early death in carriers of C allele compared to patients with the AA genotype in both Asians and Caucasians [21]. In the above meta-analysis, patients were treated with different regimens. This undoubtedly had an impact on the obtained results and can lead to deceptive conclusions. In addition, this makes it difficult to directly compare our results (only 3 studies concerned Caucasian patients treated exclusively with cisplatin / gemcitabine regimen). Interestingly, in this study, we demonstrated that in the case of response to treatment and PFS, our results are consistent with those obtained in Asian population and not in Caucasians. However, in the case of OS, our data are in conformity with literature regardless of the race of the patients [21]. Although we have taken efforts to design and perform research in comprehensive and accurate manner, our study has some limitations: population of patients enrolled in the study is heterogeneous; selected patients received not only first-line chemotherapy but also radiotherapy, whereas selected patients received subsequent lines of treatment.
In this study, we demonstrated a significantly higher risk of shortening the duration of PFS in carriers of CC genotype (2251A > C) of XPD/ERCC2 gene treated with first-line chemotherapy based on combination of cisplatin and gemcitabine. Interestingly, this is consistent with the available literature data for Asian, but not Caucasian (same as ours) patients [21].
Therefore, we believe that the conclusive answer to the question whether the SNPs of XPD/ERCC2 gene can be a useful predictor of chemotherapy based on a combination of platinum compounds and gemcitabine remains unknown. This issue requires further research on sufficiently large and homogeneous groups of patients. First of all, they should be treated with only one therapy regimen.
Conclusion
Rare CC genotype of XPD/ERCC2 gene may be considered as an unfavorable predictive factor for cisplatin/gemcitabine based chemotherapy in patients with advanced NSCLC.
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 4.2016 www.nccn.org Accessed 1 July 2017
Blumenthal GM, Karuri SW, Zhang H, Zhang L, Khozin S, Kazandjian D, Tang S, Sridhara R, Keegan P, Pazdur R (2015) Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol 33(9):1008–1014
Gridelli C, de Marinis F, Cappuzzo F, di Maio M, Hirsch FR, Mok T, Morgillo F, Rosell R, Spigel DR, Yang JCH, Ciardiello F (2014) Treatment of advanced non-small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 15:173–181
Simon GR, Somaiah N (2014) A tabulated summary of targeted and biologic therapies for non-small-cell lung cancer. Clin Lung Cancer 15:21–51
La-Beck NM, Jean GW, Huynh C et al (2015) Immune checkpoint inhibitors: new insights and current place in Cancer therapy. Pharmacotherapy 35(10):963–976
Peters GJ, Avan A, Ruiz MG, Orsini V, Avan A, Giovannetti E, Smit EF (2014) Predictive role of repair enzymes in the efficacy of cisplatin combinations in pancreatic and lung cancer. Anticancer Res 34(1):435–442
Simon GR, Ismail-Khan R, Bepler G (2007) Nuclear excision repair-based personalized therapy for non-small cell lung cancer: from hypothesis to reality. Int J Biochem Cell Biol 39(7–8):1318–1328
Sullivan I, Salazar J, Majem M, Pallarés C, del Río E, Páez D, Baiget M, Barnadas A (2014) Pharmacogenetics of the DNA repair pathways in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Lett 353(2):160–166
Simon GR, Schell MJ, Begum M, Kim J, Chiappori A, Haura E, Antonia S, Bepler G (2012) Preliminary indication of survival benefit from ERCC1 and RRM1-tailored chemotherapy in patients with advanced nonsmall cell lung cancer: evidence from an individual patient analysis. Cancer 118:2525–2531
Zhang Q, Zhu X, Zhang L, Sun S, Huang J, Lin Y (2014) A prospective study of biomarker-guided chemotherapy in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 74:839–846
Joerger M, Burgers JA, Baas P, Doodeman VD, Smits PHM, Jansen RS, Vainchtein LD, Rosing H, Huitema ADR, Beijnen JH, Schellens JHM (2012) Gene polymorphisms, pharmacokinetics, and hematological toxicity in advanced non-small-cell lung cancer patients receiving cisplatin/gemcitabine. Cancer Chemother Pharmacol 69(1):25–33
Murphy A, Chu JH, Xu M, Carey VJ, Lazarus R, Liu A, Szefler SJ, Strunk R, DeMuth K, Castro M, Hansel NN, Diette GB, Vonakis BM, Franklin Adkinson N Jr, Klanderman BJ, Senter-Sylvia J, Ziniti J, Lange C, Pastinen T, Raby BA (2010) Mapping of numerous disease-associated expression polymorphisms in primary peripheral blood CD4+ lymphocytes. Hum Mol Genet 19:4745–4757
Zhao W, Hu XJ et al (2013) Polymorphisms in the base excision repair pathway modulate prognosis of platinum-based chemotherapy in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 71(5):1287–1295
Mazzoni F, Cecere FL, Meoni G, Giuliani C, Boni L, Camerini A, Lucchesi S, Martella F, Amoroso D, Lucherini E, Torricelli F, di Costanzo F (2013) Phase II trial of customized first line chemotherapy according to ERCC1 and RRM1 SNPs in patients with advanced non-small-cell lung cancer. Lung Cancer 82:288–293
Camps C, Sarries C, Roig B, Javier Sánchez J, Queralt C, Sancho E, Martinez N, Tarón M, Rosell R (2003) Assessment of nucleotide excision repair XPD polymorphisms in the peripheral blood of gemcitabine/cisplatin-treated advanced non-small-cell lung cancer patients. Clin Lung Cancer 4(4):237–241
Liao WY, Shih JY, Chang GC, Cheng YK, Yang JCH, Chen YM, Yu CJ (2012) Genetic polymorphism of XRCC1 Arg399Gln is associated with survival in non-small-cell lung cancer patients treated with gemcitabine/platinum. J Thorac Oncol 7(6):973–981
Tibaldi C, Giovannetti E, Vasile E, Mey V, Laan AC, Nannizzi S, di Marsico R, Antonuzzo A, Orlandini C, Ricciardi S, del Tacca M, Peters GJ, Falcone A, Danesi R (2008) Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 14(6):1797–1803
de las Peñas R, Sanchez-Ronco M, Alberola V et al (2006) Polymorphisms in DNA repair genes modulate survival in cisplatin/gemcitabine-treated non-small-cell lung cancer patients. Ann Oncol 17(4):668–675
Zhang ZY, Tian X, Wu R, Liang Y, Jin XY (2012) Predictive role of ERCC1 and XPD genetic polymorphisms in survival of Chinese non-small cell lung cancer patients receiving chemotherapy. Asian Pac J Cancer Prev 13(6):2583–2586
Qin Q, Zhang C, Yang X, Zhu H, Yang B, Cai J, Cheng H, Ma J, Lu J, Zhan L, Liu J, Liu Z, Xu L, Sun X (2013) Polymorphisms in XPD gene could predict clinical outcome of platinum-based chemotherapy for non-small cell lung cancer patients: a meta-analysis of 24 studies. PLoS One 8(11):e79864
Source of Funding
Medical University of Lublin (MNmb227).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All persons gave their informed consent prior to their inclusion in the study.
Human and Animal Studies
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Disclosures
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mlak, R., Krawczyk, P., Homa-Mlak, I. et al. Predictive Value of Single Nucleotide Polymorphisms of ERCC1, XPA, XPC, XPD and XPG Genes, Involved in NER Mechanism in Patients with Advanced NSCLC Treated with Cisplatin and Gemcitabine. Pathol. Oncol. Res. 25, 1035–1045 (2019). https://doi.org/10.1007/s12253-018-0459-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-018-0459-8