Abstract
Fas Ligand (FasL) expression by cancer cells may contribute to tumour immune escape via the Fas counterattack against tumour-infiltrating lymphocytes (TILs). Whether this plays a role in colorectal carcinogenesis in Lynch syndrome was examined studying FasL expression, tumour cell apoptosis and number of TILs in colorectal neoplasms from Lynch syndrome patients (50 adenomas, 20 carcinomas) compared with sporadic cases (69 adenomas, 52 carcinomas). FasL expression was observed in 94% of Lynch syndrome adenomas and in all carcinomas. FasL expression patterns and apoptotic indices were similar in Lynch syndrome-associated neoplasms and sporadic cases. The number of TILs was higher in Lynch syndrome neoplasms than in sporadic cases. There were no correlations between FasL expression and tumour cell apoptosis or number of TILs in Lynch syndrome-associated neoplasms. So, FasL expression is an early event in Lynch syndrome and sporadic colorectal carcinogenesis, but not related to TIL number. Taken together, our data do not support a role for the Fas counterattack in colorectal carcinogenesis in Lynch syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
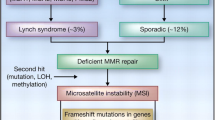
Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (HNPCC), is characterised by an autosomal dominant inheritance of early-onset colorectal cancer and an increased risk of other cancers, in particular cancer of the endometrium [1]. LS is caused by germline mutations in a mismatch repair gene, mostly hMLH1, hMSH2 and hMSH6 [1]. These genes encode proteins involved in DNA repair. Failure of the DNA mismatch repair machinery to repair errors occurring during DNA replication leads to increased length variation of simple, repetitive sequences distributed throughout the genome, so-called microsatellite instability (MSI). An international set of markers is developed to test for MSI. Tumours are scored MSI-high if at least 30% of the markers show instability, MSI-low if less than 30% show instability, or MS-stable (MSS) if none of the markers shows instability [2]. The MSI-H phenotype is found in up to 92% of colorectal cancers associated with LS and in 10–15% of sporadic colorectal cancers [1].
Dysfunction of one of the mismatch repair genes results in a rapid accumulation of genetic alterations in susceptible genes, including genes involved in growth suppression, apoptosis and signal transduction [1]. These alterations contribute to an accelerated adenoma–carcinoma sequence in LS compared to sporadic cancer. This is illustrated by the relative frequent occurrence of interval cancers between regular surveillance colonoscopies [3, 4]. In addition, LS adenomas frequently harbour a villous component and high-grade dysplasia, both markers of increased cancer risk [5, 6]. Among the genes that are targeted in the setting of MSI are BAX and caspase 5, suggesting that disturbances in apoptosis regulation may be important as a mechanism behind the accelerated colorectal carcinogenesis in LS [7, 8].
Apoptosis can be either initiated by the cell itself or by certain external stimuli. These external stimuli may induce apoptosis by targeting one of two pathways [7]. The ‘extrinsic’ pathway is initiated by triggering cell death receptors on the cell surface, leading to activation of the intracellular apoptotic machinery. The ‘intrinsic’ pathway of apoptosis is initiated via the mitochondria by cellular stress, such as chemotherapeutic drugs and radiation. Fas is a protein of the tumour necrosis factor (TNF) receptor superfamily, known to induce apoptosis through the extrinsic pathway in sensitive cells by binding to its natural ligand, Fas Ligand (FasL). In the normal human colon, all epithelial cells express Fas, but not FasL [9]. In contrast, many colorectal carcinomas and some adenomas show FasL expression [10–13]. It has been hypothesized that FasL expression in tumour cells might enable these cells to evade immune destruction by inducing apoptosis in tumour-infiltrating lymphocytes (TILs), a concept which has become known as the Fas counter-attack [14]. In a previous study, FasL expression was reported to be higher in MSI-H colorectal cancers compared to MSI-L and MSS cancers [15]. To date, FasL expression has not been examined in colorectal tumours from LS patients. We hypothesized that a diminished Fas counterattack between LS and sporadic colorectal tumours play a role as a mechanism to explain the difference in carcinogenesis. Therefore, FasL expression, tumour cell apoptosis and number of TILs in colorectal neoplasms from LS patients in comparison with sporadic cases were studied.
Materials and Methods
Patients
Lynch Syndrome
At the University Medical Centre Groningen, patients with a germline mismatch repair gene mutation and/or fulfilling the Amsterdam II criteria undergo colonoscopy every 1–2years starting at the age of 25years, according to the current surveillance guidelines [1]. All colorectal adenomas and carcinomas removed in these patients over a period of 23years with sufficient material available to allow serial sectioning for immunohistochemistry were selected for this study. In total, 20 carcinomas were examined, 17 from proven mutation carriers (8 hMLH1, 7 hMSH2, 2 hMSH6), as determined by sequencing of genomic DNA, and three from patients with a family history fulfilling the Amsterdam II criteria. Fifty adenomas, 23 from proven mutation carriers, 27 from patients with a family history fulfilling the Amsterdam II criteria, were analyzed.
Sporadic cases
The control group consisted of sporadic carcinomas from a previously reported cohort of 500 patients with stage III colon cancer [16]. From this cohort, in which MSI analysis has been performed, we selected all MSI-H tumours (n = 26) and randomly 26 MSS tumours. MSI-H tumours were included to determine possible differences between sporadic MSI-H tumours and LS-associated tumours which also are characterized by the MSI-H phenotype. None of the 26 patients with a MSI-H tumour was known with a MMR gene mutation or had a family history fulfilling the Amsterdam II criteria, so these were considered truly sporadic cases. Sporadic adenomas of which sufficient material was available to allow serial sectioning for immunohistochemical staining were selected from a previously described series [17] as control adenomas (n = 69).
Histologic classifications were carried out on haematoxylin and eosin stained slides. The morphological classification of the carcinomas and adenomas was conducted according to the World Health Organization (WHO) criteria [18]. Adenocarcinomas were graded into well, moderately and poorly differentiated. For statistical purposes, tumours with good and moderate differentiation were compared with tumours with poor differentiation. From adenomas, the circumferential size was measured, and the severity of dysplasia was expressed as low- or high-grade. Adenomas were classified as tubular, tubulovillous or villous [18]. For statistical purposes, tubulovillous and villous adenomas were taken together.
Immunohistochemistry
For immunohistochemical staining for FasL, sections were deparaffinized in xylene, rehydrated through decreasing concentrations of alcohol and finally in phosphate-buffered saline. For antigen retrieval, slides were subjected to steam-heat for 30min, using a commercially available steamer. After washing with PBS, sections were incubated with avidin and biotin blocking solutions (Vector Laboratories, Burlingame, CA, USA), followed by incubation for 1h at room temperature with a monoclonal anti-FasL antibody (MAb33, Transduction Laboratories, Lexington, KY, USA) in a 1:300 dilution. After washing with PBS, slides were incubated with a 1:300 dilution of a biotinylated rabbit-anti-goat antibody (DAKO, Glostrup, Denmark), followed by addition of streptavidin-conjugated peroxidase (DAKO). Peroxidase activity was visualized with diaminobenzidine. Counterstaining was performed with haematoxylin. As negative controls, slides were immunostained in the absence of the primary antibody, and in these cases no immunostaining was detected. Evaluation of staining was performed by two independent investigators, without knowledge of the histopathological data. The percentage of positive cells was estimated and scored on a semiquantitative scale as (1) 0–25%, (2) 26–50%, (3) 51–75%, (4) 76–100%, in accordance with others [14, 15].
Apoptosis in epithelial cells was determined by immunohistochemistry with the murine monoclonal antibody M30 (Boehringer Mannheim, Mannhein, Germany), which reacts with a cleavage product of cytokeratin 18, released by activated caspase [19]. The immunoreactivity of the M30 antibodies is confined to the cytoplasm of apoptotic cells and is present during early apoptosis [20]. M30 staining has been shown to correlate well with the gold standard of morphological criteria [17]. Staining procedures for M30 were performed as described before [17]. Apoptosis was assessed in at least 1,000 tumour cells and expressed as a percentage of the total number of cells counted (apoptotic index). M30 positivity was identified as brown cytoplasmic staining.
The presence of TILs was assessed using a monoclonal antibody against CD8. Antigen retrieval was performed by immersing slides in 1mM EDTA buffer (pH 8.0) and heating in a microwave oven for 8min. The primary antibody (DAKO) was applied in a 1:50 solution for 1h followed by a secondary rabbit-anti-mouse antibody conjugated with peroxidase (DAKO) and a tertiary goat-anti-rabbit peroxidase-conjugated antibody.
For each tumour, the number of intraepithelial CD8+ cells was determined in ten high power fields (400 × magnification), in accordance with other studies [15, 21, 22]. TILs were defined as intraepithelial rather than peri-tumoural or stromal lymphocytes.
Statistical Analysis
Statistical significance of differences between LS and sporadic cases was tested using the Mann-Whitney test for continuous variables and chi-square tests for discontinuous variables. To test the relationship between increasing FasL expression and continuous variables as apoptotic index and CD8 counts, the Wilcoxon rank sum test was used. P-values <0.05 were considered significant. SPSS for Windows software (SPSS Inc., Chicago, IL, USA) was used.
Results
Tumour Characteristics
Patient and tumour characteristics are summarized in Table 1. Inherent to the patient groups, several differences were observed between groups. Mean age of LS patients at the time of adenoma removal was lower than for those with sporadic disease (p < 0.0001). LS-associated adenomas were smaller (p = 0.002), more often containing high-grade dysplasia (p < 0.01) and more often proximally localized (p < 0.05), i.e. proximal to the splenic flexure, compared with their sporadic counterparts. LS-associated carcinomas and MSI-H sporadic carcinomas were more often localized in the proximal colon compared to sporadic MSS cases, (p < 0.001).
FasL Expression in LS and Sporadic Colorectal Neoplasms
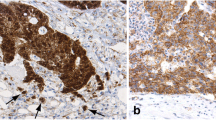
Staining results are summarized in Table 2. FasL expression in epithelial cells was seen in 47/50 LS-associated adenomas and 64/69 sporadic adenomas (not significantly different). FasL expression was cytoplasmic and generally heterogeneously distributed within adenomatous tissue. In both groups, approximately half of the samples displayed expression in more than 50% of tumour cells. The extent of FasL positivity was not related to age, gender, adenoma size, location, degree of dysplasia or villous architecture, neither in LS-associated, nor in sporadic cases.
In carcinomas, FasL expression was present in all samples investigated. FasL expression was generally heterogeneously distributed. Percentages of positively staining cells were similar in LS, MSS and MSI-H sporadic tumours. In all three groups, approximately 80% of cases demonstrated FasL expression in more than 50% of cells. FasL expression was not related to age, gender, tumour location or degree of differentiation. When comparing FasL expression in adenomas and carcinomas, it was similar in LS-associated neoplasms (Fig. 1). In sporadic cases, percentages of positively staining cells were higher in carcinomas than in adenomas (p < 0.001). This was true for MSS carcinomas as well as for MSI-H carcinomas.
Tumour Cell Apoptosis and Number of TILs in LS and Sporadic Colorectal Neoplasms
The level of tumour cell apoptosis, assessed by M30 immunoreactivity (Fig. 2), was similar between LS and sporadic adenomas, although there was a non-significant trend towards higher levels in LS adenomas (p = 0.08). The same trend was observed in LS carcinomas, with apoptotic indices being slightly higher than in sporadic cancers (p = 0.07). When comparing carcinomas with adenomas, mean apoptotic indices were higher in carcinomas, both in LS (p < 0.05) and in sporadic cases (p < 0.001).
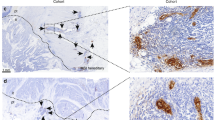
The number of TILs, assessed by CD8 positivity, was higher in LS adenomas compared with sporadic adenomas (p < 0.05, Fig. 3). Within the group of LS adenomas, the mean number of TILs was higher in adenomas with high-grade dysplasia compared with those with low-grade dysplasia (8.8 ± 1.8 vs 4.7 ± 0.8, p < 0.001). The mean number of TILs was also higher in adenomas with proximal location compared with those with distal location (9.4 ± 1.9 vs 4.7 ± 0.8, p < 0.001). The mean number of TILs was not correlated with adenoma size or the presence of villous architecture in LS adenomas. In sporadic adenomas, the number of TILs was not correlated with adenoma size, degree of dysplasia, architecture or location.
Staining of tumour-infiltrating lymphocytes (TILs) by CD8 immunoreactivity (brown). A, Lynch syndrome associated cancer; B, Sporadic MSS cancer; C, Lynch syndrome associated adenoma; D, Sporadic adenoma. The number of TILs is higher in cases associated with Lynch syndrome compared to sporadic ones (magnification × 400)
In carcinomas, the number of TILs was similar in LS-associated carcinomas and MSI-H sporadic cancers but higher in both these groups than in MSS sporadic tumours (p = 0.01 and 0.02 respectively, Fig. 3). In LS-associated tumours, TIL density was higher in carcinomas than in adenomas (p < 0.001). There was no correlation between the number of TILs and tumour location or degree of differentiation. This was the case in LS-associated cancers as well as in sporadic cancers.
Relationship Between FasL Expression, Tumour Cell Apoptosis and TILs
To search support for the FasL counterattack hypothesis, we determined whether higher FasL expression in neoplasms was associated with lower levels of tumour cell apoptosis or reduced TIL densities (Table 3). For this analysis, adenomas and carcinomas from LS patients and were taken together as well as adenomas and MSS carcinomas from sporadic origin. In LS-associated cases, there were no positive or negative correlations between the percentage of FasL positivity on the one hand and levels of tumour cell apoptosis or number of TILs on the other. There was also no correlation between the degree of tumour cell apoptosis and the number of TILs. In contrast, in sporadic cases, there was a positive correlation between higher FasL expression and degree of tumour cell apoptosis (Spearman’s rho correlation coefficient 0.27, p = 0.008). There was no correlation between FasL expression and the number of TILs, nor between the degree of tumour cell apoptosis and the number of TILs in sporadic neoplasms.
Discussion
In this study, we sought to determine whether the Fas counterattack plays a role in the accelerated colorectal carcinogenesis in LS patients compared to sporadic disease. We found no support for such a mechanism, as FasL expression was similar between LS and sporadic tumours. In addition, there were no correlations between the percentages of FasL positivity and the number of TILs. A novel finding from our study was that LS-associated adenomas, in addition to LS-associated carcinomas, are also characterized by higher numbers of TILs compared to sporadic cases.
Colorectal carcinogenesis in LS is believed to be accelerated when compared with sporadic cases. This is illustrated by the relative frequent occurrence of interval cancers between regular surveillance colonoscopies. In addition, LS-associated adenomas frequently harbour villous components and high-grade dysplasia, which both are markers of increased cancer risk. Differences between LS and sporadic colorectal neoplasms have been extensively studied, demonstrating differences in molecular genetic alterations [23–26] and histopathological characteristics [6, 15, 27, 28]. The accelerated carcinogenesis in LS does not seem to be reflected by a difference in degree of apoptosis with sporadic carcinogenesis [29, 30], although several differences in expression of apoptosis-regulating proteins have been found [30, 31, 32]. To our knowledge, FasL expression has not been studied before in LS-associated neoplasms. Our results indicate similar expression patterns of FasL in LS and sporadic neoplasms. So, FasL expression seems to occur early in colorectal carcinogenesis, irrespective of the sporadic or hereditary origin of the lesions.
We found that FasL expression was more prevalent in carcinomas than in adenomas, consistent with other reports [10–13]. It was recently demonstrated that MSI-H tumours were associated with a reduced frequency of FasL expression relative to non-MSI-H tumours [33]. In our study, a similar trend was seen although this did not reach statistical significance.
Infiltration of lymphocytes is a prominent feature of MSI-H tumours, including LS-associated colorectal cancer [21, 22, 27, 34]. This was confirmed in the present report. In this study, we show for the first time that LS-associated adenomas display the same feature. Moreover, we found that the mean number of TILs was higher in LS adenomas with high-grade dysplasia compared with those with low-grade dysplasia. Only few studies are available that have evaluated the degree of infiltration with lymphocytes in adenomas [35–37]. From these studies, it appears that the number of TILs increases in the course of the adenoma-carcinoma sequence, which is corresponding to our results. It has also been shown that the number of TILs is an independent prognostic factor in colorectal cancer [38–40]. As LS-associated colorectal cancer and sporadic MSI-H colorectal cancer are associated with better survival rates than sporadic cases [1], it has been suggested that the presence of a cytotoxic anti-tumour immune response may at least in part contribute to the survival advantage described in these patients [34, 38]. Whether TILs in LS-associated and MSI-H sporadic tumours are really activated cytotoxic T-cells remains to be demonstrated.
The aim of our study was to determine whether higher FasL expression in neoplasms was associated with lower levels of tumour cell apoptosis or increased levels of apoptotic TILs, reflected by reduced TIL density. We found no such correlations in LS-associated neoplasms. In sporadic cases, a positive rather than a negative correlation between FasL expression and tumour cell apoptosis was found. This finding may be a consequence of elevated FasL levels triggering tumour cell apoptosis in a manner similar to autocrine suicide of activated T-cells [41]. Our findings are in contrast with those from Houston et al, who did not find a correlation between FasL expression and degree of tumour cell apoptosis [42]. It must be noted that their series consisted of only ten colorectal tumours, which may explain the absence of a correlation in their study. The same research group recently found a non-significant negative association between intensity of FasL staining and number of TILs in a series of 91 colon carcinomas [43]. Two studies reported a positive correlation between FasL expression and apoptosis in TILs [43, 44], whereas we did not find an association between FasL expression and TIL density. The background for the discrepancy between these data and ours is unclear. A possible explanation may lie in the fact that in the other studies TUNEL and ISNT were used to assess TIL apoptosis. Both these techniques have poor specificity for apoptosis and their use is currently discouraged [7, 45].
The question whether the Fas counterattack plays a role at all in colorectal carcinogenesis is surrounded with controversy [46, 47]. The idea behind the concept is that FasL expression by tumour cells may enable those cells to kill anti-tumour immune effector T-cells that infiltrate the tumour. Many tumours and tumour cell lines of varying histologic origin express FasL [48]. Several functional studies have shown that FasL positive colon tumour cells can induce apoptosis of Fas-sensitive lymphocytes in vitro [49, 50]. In addition, inhibition of FasL expression reduced tumour development and growth in a xenograft experiment [51]. There are however also experimental data which seriously question the concept of the Fas counterattack. In a replication study, FasL-expressing colon cancer cells did not induce apoptosis in Fas-sensitive target cells [52]. In a gene transfer experiment, Fas resistant colon cancer cells, subcutaneously injected into mice, rapidly regressed upon transfection with FasL [53]. In another experiment, cytotoxic T-cells were not sensitive to FasL displayed on the surface of antigen-presenting cells [54]. Taken together, the role of the Fas counterattack in colorectal carcinogenesis is at least questionable [48].
In conclusion, we observed upregulation of FasL during colorectal carcinogenesis, both in LS-associated and in sporadic cases, without a correlation with the number of TILs. Our data do not support an important role for the Fas counterattack as a mechanism behind the difference between colorectal carcinogenesis in LS-associated and sporadic cases.
Abbreviations
- FasL:
-
Fas Ligand
- TIL:
-
tumour-infiltrating lymphocytes
- LS:
-
Lynch syndrome
- HNPCC:
-
hereditary nonpolyposis colorectal cancer
- MSI:
-
microsatellite instability
- MSS:
-
microsatellite stable
- TNF:
-
tumour necrosis factor
- WHO:
-
World Health Organization
References
Hendriks YM, de Jong AE, Morreau H et al (2006) Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 56:213–225
Boland CR, Thibodeau SN, Hamilton SR et al (1998) A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
De Vos tot Nederveen Cappel WH, Nagengast FM, Griffioen G et al (2002) Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon Rectum 45:1588–1894
Vasen HF, Nagengast FM, Khan PM (1995) Interval cancers in hereditary nonpolyposis colorectal cancer (Lynch syndrome). Lancet 345:1183–1184
Ponz de Leon M, Della CG, Benatti P et al (1998) Frequency and type of colorectal tumors in asymptomatic high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev 7:639–641
Rijcken FE, Hollema H, Kleibeuker JH (2002) Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut 50:382–386
Koornstra JJ, de Jong S, Hollema H et al (2003) Changes in apoptosis during the development of colorectal cancer: a systematic review of the literature. Crit Rev Oncol Hematol 45:37–53
Woerner SM, Kloor M, von Knebel Doeberitz M et al (2006) Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark 2:69–86
Möller P, Koretz K, Leithäuser F et al (1994) Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer 57:371–377
Belluco C, Esposito G, Bertorelle R et al (2002) Fas ligand is up-regulated during the colorectal adenoma-carcinoma sequence. Eur J Surg Oncol 28:120–125
Bennett MW, O’Connell J, Houston A et al (2001) Fas ligand upregulation is an early event in colonic carcinogenesis. J Clin Pathol 54:598–604
Shimoyama M, Kanda T, Liu L et al (2001) Expression of Fas ligand is an early event in colorectal carcinogenesis. J Surg Oncol 76:63–68
Younes M, Schwartz MR, Finnie D et al (1999) Overexpression of Fas ligand (FasL) during malignant transformation in the large bowel and in Barrett’s metaplasia of the esophagus. Hum Pathol 30:1309–1313
O’Connell J, Bennett MW, O’Sullivan GC et al (1998) Fas ligand expression in primary colon adenocarcinomas: evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J Pathol 186:240–246
Michael-Robinson JM, Pandeya N, Cummings MC et al (2003) Fas ligand and tumour counter-attack in colorectal cancer stratified according to microsatellite instability status. J Pathol 201:46–54
van Geelen CM, Westra JL, de Vries EG et al (2006) Prognostic significance of tumor necrosis factor-related apoptosis-inducing ligand and its receptors in adjuvantly treated stage III colon cancer patients. J Clin Oncol 24:4998–5004
Koornstra JJ, Rijcken FE, de Jong S et al (2004) Assessment of apoptosis by M30 immunoreactivity and the correlation with morphological criteria in normal colorectal mucosa, adenomas and carcinomas. Histopathology 44:9–17
Jass JR, Sobin LH, Watanabe H (1990) The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition, Cancer 66:2162–2167
Caulin C, Salvesen GS, Oshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138:1379–1394
Leers MP, Kolgen W, Bjorklund V et al (1999) Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 187:567–572
Dolcetti R, Viel A, Doglioni C et al (1999) High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol 154:1805–1813
Smyrk TC, Watson P, Kaul K et al (2001) Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91:2417–2422
Deng G, Bell I, Crawley S et al (2004) BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res 10:191–195
Johnson V, Volikos E, Halford SE et al (2005) Exon 3 beta-catenin mutations are specifically associated with colorectal carcinomas in hereditary non-polyposis colorectal cancer syndrome. Gut 54:264–267
Losi L, Fante R, Di Gregorio C et al (1995) Biologic characterization of hereditary non-polyposis colorectal cancer. Nuclear ploidy, AgNOR count, microvessel distribution, oncogene expression, and grade-related parameters. Am J Clin Pathol 103:265–270
Yagi OK, Akiyama Y, Nomizu T et al (1998) Proapoptotic gene BAX is frequently mutated in hereditary nonpolyposis colorectal cancers but not in adenomas. Gastroenterology 114:268–274
Jass JR (2004) HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer 3:93–100
Young J, Simms LA, Biden KG et al (2001) Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol 159:2107–2116
Heijink DM, Kleibeuker JH, Jalving M et al (2007) Independent induction of caspase-8 and cFLIP expression during colorectal carcinogenesis in sporadic and HNPCC adenomas and carcinomas. Cell Oncol 29:409–419
Rijcken FE, Koornstra JJ, van der Sluis T et al (2007) Early carcinogenic events in HNPCC adenomas: differences with sporadic adenomas. Dig Dis Sci [Epub ahead of print]
Sinicrope FA, Roddey G, Lemoine M et al (1998) Loss of p21WAF1/Cip1 protein expression accompanies progression of sporadic colorectal neoplasms but not hereditary nonpolyposis colorectal cancers. Clin Cancer Res 4:1251–1261
Sinicrope FA, Lemoine M, Xi L et al (1999) Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology 117:350–358
Houston AM, Michael-Robinson JM, Walsh MD et al (2008) The “Fas counterattack” is not an active mode of tumor immune evasion in colorectal cancer with high-level microsatellite instability. Hum Pathol 39:243–250
Michael-Robinson JM, Biemer-Huttmann A, Purdie DM et al (2001) Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 48:360–366
Jackson PA, Green MA, Marks CG et al (1996) Lymphocyte subset infiltration patterns and HLA antigen status in colorectal carcinomas and adenomas. Gut 38:85–89
Rubio CA, Jacobsson B, Castaños-Velez E (1999) Cytotoxic intraepithelial lymphocytes in colorectal polyps and carcinomas. Anticancer Res 19(4B):3221–3227
Warabi M, Kitagawa M, Hirokawa K (2000) Loss of MHC class II expression is associated with a decrease of tumor-infiltrating T cells and an increase of metastatic potential of colorectal cancer: immunohistological and histopathological analyses as compared with normal colonic mucosa and adenomas. Pathol Res Pract 196:807–815
Guidoboni M, Gafà R, Viel A et al (2001) Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol 159:297–304
Naito Y, Saito K, Shiiba K et al (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Ropponen KM, Eskelinen MJ, Lipponen PK et al (1997) Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182:318–324
Dhein J, Walczak H, Baumler C et al (1995) Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373:438–441
Houston A, Waldron-Lynch FD, Bennett MW et al (2003) Fas ligand expressed in colon cancer is not associated with increased apoptosis of tumor cells in vivo. Int J Cancer 107:209–214
Houston A, Bennett MW, O’Sullivan GC et al (2003) Fas ligand mediates immune privilege and not inflammation in human colon cancer, irrespective of TGF-beta expression. Br J Cancer 89:1345–1351
Okada K, Komuta K, Hashimoto S et al (2000) Frequency of apoptosis of tumor-infiltrating lymphocytes induced by fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res 6:3560–3564
Walker JA, Quirke P (2001) Viewing apoptosis through a TUNEL. J Pathol 195:275–276
O’Connell J, Houston A, Bennett MW, O’Sullivan GC, Shanahan F (2001) Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med 7:271–274
Restifo NP (2000) Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med 6:493–495
Sträter J, Möller P (2003) CD95 (Fas/APO-1)/CD95L in the gastrointestinal tract: fictions and facts. Virchows Arch 442:218–225
Huber V, Fais S, Iero M et al (2005) Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 128:1796–1804
O’Connell J, O’Sullivan GC, Collins JK et al (1996) The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 184:1075–1082
Ryan AE, Shanahan F, O’Connell J et al (2005) Addressing the “Fas counterattack” controversy: blocking fas ligand expression suppresses tumor immune evasion of colon cancer in vivo. Cancer Res 65:9817–9823
Favre-Felix N, Fromentin A, Hammann A et al (2000) Cutting edge: the tumor counterattack hypothesis revisited: colon cancer cells do not induce T cell apoptosis via the Fas (CD95, APO-1) pathway. J Immunol 5023–5027
Arai H, Gordon D, Nabel EG et al (1997) Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA 94:13862–13867
Jekle A, Obst R, Lang F et al (2000) CD95/CD95 ligand-mediated counterattack does not block T cell cytotoxicity. Biochem Biophys Res Commun 272:395–399
Acknowledgements
This study was supported by the Dutch Cancer Society, grant RUG 1998–1660 and the Dutch Digestive Diseases Foundation, grant 2001–31.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Koornstra, J.J., de Jong, S., Boersma-van Eck, W. et al. Fas Ligand Expression in Lynch Syndrome-Associated Colorectal Tumours. Pathol. Oncol. Res. 15, 399–406 (2009). https://doi.org/10.1007/s12253-008-9136-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9136-7