Abstract
Purpose
As Rosa damascena essential oils (RDEOs) have antioxidant, antibacterial, antiviral, and insecticidal activity, they could therefore be useful in the treatment of breast cancer. In the current study, an attempt was made to incorporate RDEO in a lipid-based drug delivery system, namely, nanostructured lipid carrier (NLC) to boost its anticancer effect compared to cisplatin.
Methods
Gas chromatography (GC) identified the chemical compositions of RDEO. RDEO-NLCs were prepared using the probe ultrasonication method. The obtained nanoparticles were characterized in terms of particle size, polydispersity index, and zeta potential by dynamic light scattering. The encapsulation efficiency of the formulations and their loading capacity were also determined, and transmission electron microscopy (TEM) was employed to evaluate the morphology of the optimal formulation (quoted as RDEO-NLC2). The anticancer effect of RDEO-NLC2 on MDA-MB-231 cells and apoptosis were assessed using MTT and in vitro cellular assays respectively.
Results
TEM result revealed a distinct spherical shape for RDEO-NLC2, with an average particle size of 78.39 ± 1.5 nm obtained by Zetasizer. The results also showed that the obtained particles had a negative surface charge (− 31.0 mV) with a polydispersity index of 0.28 ± 0.01. The chemotherapy drug cisplatin showed more cytotoxicity than RDEO-NLC2 against cancer cells. Cellular data demonstrated that RDEO-NLC2 like cisplatin can decline the viability of MDA-MB-231 cells through apoptosis compared to cells treated with the placebo and free RDEO.
Conclusion
RDEO-NLC2 has the ability to stimulate apoptosis in the human BC cell line MDA-MN-231; hence, it can be beneficial in the treatment of patients suffering from breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the last century, breast cancer (BC) has been one of the most prevalent cancers and the second major cause of cancer death among women globally [1]. It is therefore crucial to explore anticancer formulations with better performance and fewer side effects. Based on the presence of molecular markers or their absence for estrogen or progesterone receptors or based on human epidermal growth factor 2 (ERBB2), BC is subdivided into three major types: hormone receptor-positive/ERBB2 negative, ERBB2 positive, and triple-negative, accounting for 70%, 15–20%, and 15% respectively of BC patients [2].
Natural products are becoming popular in the use of anticancer formulations due to safety, efficiency, accessibility, anticancer properties, and resistance-overcoming activities [3]. Plants have the ability to produce chemical anticancer compounds [4] with biological activities that can improve human health [5]. Rosa damascena (R. damascena; Damask rose), an important species of the genus Rosa, comprises at least 200 species. Many studies have shown that this plant contains many chemical compositions with anticancer activity [6,7,8,9]. The essential oil (EO) of R. damascena, compared to other rose types, has been shown to have the highest economic value for application in various industries, including the pharmaceutical industry [9, 10]. The antidiabetic, antioxidant, antimicrobial, anti-aging, anti-inflammatory, and anti-HIV effects of this plant have been well documented [10, 11]. EOs possess biological activities viz antioxidant, antibacterial, antiviral, and insecticidal activity which are used in the treatment of cancer [12]. As EOs are unstable, volatile, and easily degradable, incorporation of EOs in formulations could be challenging. It has been shown that the encapsulation of EOs in nanosystems could be a useful approach for increasing their stability and improving their antibacterial activity and targeted delivery [13, 14].
Nanocarriers have been demonstrated to remarkably ameliorate the therapeutic effects of anticancer drugs. Nanocarriers are capable of boosting the efficiency and safety of drugs by enhancing their stability and water solubility, improving their uptake by targeted cancer cells, increasing their circulation time, or inhibiting their enzyme degradation [15]. Nanostructured lipid carriers (NLCs) are lipid-based nanoparticulate drug delivery systems comprising solid and liquid lipids. The main advantages of these nanocarriers are that they are capable of high drug loading and an increase in drug encapsulation [16]. NLCs also benefit from many other unique advantages for in vivo applications such as easy binding to target ligands incorporation of multiple drug molecules (combinatorial therapy) and minimal drug resistance [17].
Cisplatin is the first and one of the most important metal-based chemotherapeutic drugs with biological activity. It can bind to genomic or mitochondrial DNA to induce DNA lesions; arrest DNA replication; inhibit the creation of DNA, mRNA, and proteins; and activate varied transduction pathways, which ultimately leads to necrosis or apoptosis. However, due to unwanted effects and drug resistance, cisplatin does not represent its maximum potential. Resistance of cancer cells to cisplatin is dependent on several factors such as decreased drug accumulation, drug inactivation by binding to various proteins, increased DNA repairing, and protein alterations that show apoptosis. Although treatment with cisplatin could be very beneficial in the treatment of BC, it shows major toxicities such as ototoxicity, nephrotoxicity, and hepatotoxicity [18].
Cancer chemoprevention is an approach that uses synthetic, pharmaceutical, or natural compounds to prevent the process of carcinomas. While the use of conventional chemotherapy is the most common treatment method for cancer, the application of anticancer drugs in this way has some limitations [1]. Moreover, this method often induces multidrug resistance in cancerous cells. Therefore, the development of efficient cancer treatment strategies has significant research importance and clinical value in the efficient treatment of cancer patients [19]. Given the complications of the chemical drug, using nanocarriers for the delivery of medicinal plant compounds in nanoparticle formulations seems to be beneficial for cancer treatment. This study was therefore undertaken to characterize and optimize R. damascena EO NLC and to assess the anticancer effects and appropriateness of the optimized R. damascena NLC preparation (RDEO-NLC2) in the human BC cell line, MDA-MB-231, in comparison to cisplatin.
Materials and Methods
Materials
Rosa damascena essential oil (RDEO; batch number 63014070) was procured from Barij Essence Pharmaceutical Co. (Iran). Span 60 (Sn60), Span 80 (Sn80), glycerol monostearate (GMS), Tween 80 (Tn80), oleic acid (OA), and stearic acid (SA) were all obtained from Merck (Germany). Milli-Q system (Millipore, Direct-Q, Bedford, MA, USA) was used for the purification of distilled water. The chemotherapy drug, cisplatin, was obtained from Mylan Pharmaceutical Co. (White Sulphur Springs, West Virginia, USA). Fetal bovine serum (FBS) and Dullbecco’s Modified Eagle Medium (DMEM) were both purchased from Gibco (Germany). Trypsin and the 3-(4,5-dimethylthiazol-2-yl) 2,5- diphenyltetrazolium bromide (MTT), as well as the annexin V/PI (apoptosis detection kit), were acquired from Exbio Co. (Czech Republic).
Gas Chromatography (GC)-Mass Spectrometry (MS) Analysis
The assessment of R. damascena’s composition was carried out by GC, and its chromatographic analysis was performed using the GC-MS Agilent GC7890-MS5975 and a computer equipped with the Wiley 7n.l library. At first, 100 µl of the EO of R. damascena was collected with an autosampler and decanted into a glass vial with a volume of 2 cc. The sodium sulfate salt was added to the vial for dehydration. After the addition of 900 µl of hexane solvent to the vial, its lid was placed on and shaken until a transparent solution was attained. The resultant solution was then injected into the HP-5MS column with a length of 30 m and a diameter of 0.25 mm. Helium (1 ml/min) as the carrier gas was used. The temperature was adjusted at 60 °C for 4 min, followed by 3 °C/min to 100 °C for 2 min and then 4 °C/min to 250 °C for 5 min. In the end, the sample (1 µl) was injected with the split injection technique (ratio 50:1).
Preparation of RDEO-NLC
Based on the probe ultrasonication method, various formulations of RDEO-NLCs were prepared [20]. In order to prepare NLCs, various lipids and surfactants were tested, and those that were able to dissolve RDEO were selected (e.g., SA, OA, GMS, Tn80, and Span 60/Span 80). The selection of the aforementioned surfactants and their respective ratios was based on the preliminary results previously published by the authors in another publication. These prior findings provided valuable insights and guided the rational choice of surfactants for the current study [21]. First, hydrophobic surfactants (0.1% w/w; Span 60 or Span 80), OA (0.03%), SA, and GMS were mixed, and the mixture was heated up to 82 °C to melt lipids. After obtaining a uniform mixture, RDEO (0.1 g) plus 2/3 of the aqueous solution comprising 0.2 g hydrophilic surfactant (Tn80) was added to the molten lipid phase. The mixture was kept at ~ 82 ℃ under probe sonication (Topsonics, Iran) for 5 min. At this stage, a coarse pre-emulsion can be formed. The final mixture was placed in an ice bath to reduce the temperature followed by the addition of the remaining 1/3 of the hydrophilic surfactant solution. The final mixture was then sonicated for a further 10 min. A placebo without RDEO was also made for comparison purposes. Finally, different formulations were developed based on the information provided in Table 1. RDEO-NLC2 was selected based on encapsulation efficiency, particle size, and zeta potential (ZP) for further studies.
Physiochemical Characterization of NLCs
The obtained NLC formulations were characterized in terms of polydispersity index (PDI), particle size, and zeta potential (ZP) by employing dynamic light scattering (DLS and Zetasizer Nano ZS, Malvern Instruments, UK) respectively. To observe the low-intensive scattered light signals from the smaller particles, a light detector was used. To this end, a scattered light detector was adjusted at an angle of 173° (non-invasive backscatter) at 25 °C. The sample was diluted in highly purified water before PDI, particle size, and ZP of the samples can be determined (n = 3) using a Zetasizer. Table 1 depicts the achieved results.
Encapsulation Efficiency (EE) of NLC and Its Loading Capacity
A centrifugation technique was used to determine the EE of the NLCs. The RDEO-containing NLCs were isolated from the unencapsulated materials by centrifugation (laboratory centrifuge Hettich Universal 320) at 10,000 rpm for 10 min. After destroying the NLCs in ethanol 90%, the encapsulated content of RDEO at a wavelength of 270 nm was determined by employing a UV/VIS spectrophotometer. Equations 1 and 2 were employed to calculate the EE percentage and the loading capacity (LC) percentage:
In the equations, Wa, WL, and Ws denote the weight of RDEO, the mass of lipids added to produce nanoparticles, and the weight of RDEO in the supernatant, respectively [21].
Transmission Electron Microscope (TEM) Analysis
TEM is a microscopic technique that determines the morphology and surface behavior of nanoparticles and nanoformulations [22]. TEM (Carl Zeiss Meditec AG, Jena, Germany) was used to study the morphology of RDEO-NLC2 [23]. The RDEO-NLC2 samples were first diluted, and then, one drop of the diluted sample was located onto a 200-mesh carbon-coated copper grid. Subsequently, the diluted sample was stained with a 2% phosphotungstic acid solution and allowed to dry at room temperature (≈ 23 ℃) before capturing the image.
Cell Culture
The MDA-MB-231 cell line acquired from the Pasteur Institute of Iran (Tehran) was cultured in DMEM (Gibco, USA) containing 10% FBS (Gibco), penicillin (100 U/ml), and streptomycin (100 µg/ml). A humid environment of 5% CO2 in a 37 °C incubator (Memmert, Germany) was used to preserve the cells before using them in the cell viability test.
Cell Viability Assay
MDA-MB-231 has been used in triple-negative breast cancer (TNBC studies) [24, 25] in the assessment and efficiency of nanoparticles. This was the rationale for the selection of this cell line for this research. In this assay, by the action of mitochondrial reductase, the water-soluble yellow dye MTT was changed to an insoluble purple formazan. The viability of MDA-MB-231 cells in the presence of cisplatin, RDEO-NLC2, placebo, and RDEO was assessed by MTT assay as previously explained elsewhere [26]. In brief, formazan was solubilized, and its concentration was measured by optical density at 570 nm wavelength. Afterward, the cell line MDA-MB-231 (9000 cells per well based on the constructed standard curve) was cultivated in 96-well plates and incubated overnight (24 h). Next, the cells were treated with 6.25, 12.5, 25, 50, and 100 ppb (part per billion) concentrations of each of RDEO, RDEO-NLC2, and placebo (NLC) and of cisplatin (1, 5, 10, 20, and 50 µg/ml) for chemotherapy at 37 °C for 24 h and 48 h. The cells as a control were established in DMEM consisting of 10% FBS. After the above-mentioned times, the medium was washed with PBS, and then, a new medium comprising MTT solution was incorporated into the cells at 37 °C for 4 h. When the unreduced MTT and medium were removed, 100 µl of DMSO was added to each well to dissolve the MTT formazan crystal at room temperature (≈ 23 ℃). After shaking the plates manually for 2 min, the formazan product absorbance was documented using a microplate reader (BioTek Instruments, Inc., Winooski, USA) at a wavelength of 570 nm. Ultimately, the cell viability was determined based on Eq. 3 [21].
- OD:
-
is the optical density.
Apoptosis Detection by Flow Cytometry
Apoptosis is a common form of cell death in eukaryotes [27]. Flow cytometry is utilized for detecting and quantifying apoptosis in mammalian cells [28]. By seeding MDA-MB-231 cells onto six-well plates in a DMEM medium comprising 10% FBS, the rate of apoptosis was determined. For adhesion, the aforesaid cells were incubated at room temperature for 24 h. The cells were then treated with half-maximal inhibitory concentration (IC50) of RDEO-NLC2, placebo, and cisplatin for 24 and 48 h. In the next step, the cells were first detached and rinsed in PBS, then resuspended in annexin V, and finally stained with both annexin V and propidium iodide for 15 min. In the last step, using flow cytometry, all the cells to measure the apoptotic cell proportion were analyzed with the aid of the FlowJo software (TreeStar Inc., San Carlo, CA, USA).
Statistical Analysis
The obtained data were statistically evaluated by employing a two-way analysis of variance (ANOVA; SPSS, version 19.0; SPSS Inc., Chicago, IL, USA). ANOVA was also used to compare the treated and control groups. The level of probability (p) was used to be statistically significant at (p < 0.05).
Results and Discussion
It has been shown that the formation of hydrophobic drug-loaded nanomedicines not only improves their stability, solubility, bioavailability, biodistribution, pharmacokinetics, and pharmacodynamics, but also brings about a decline in the toxicity of the drug [29, 30]. The obtained results in terms of the extract composition, formulation characterization, and the anticancer effect of the obtained nanoparticles are discussed below.
GC-MS Analysis
After analyzing the RDEO extracts by GC-MS, 39 major compounds with their respective peak areas were found. By comparing the mass fragmentation patterns of comparable compounds obtained for the extract with the data available in the WILEY library, different compounds were identified and listed in Table 2. The most important compounds were beta-citronellol (31.91%), nonadecane (21.43%), heneicosane (11.80%), geraniol (9.86%), 9-nonadecene (4.17%), eicosane (2.76%), heptadecane (2.27%), tricosane (2.12%), germacrene-D (1.81%), methyleugenol (1.65%), nerol (1.16%), linalool (0.83%), alpha.-guaiene (0.68%), 1,8-cineole (0.64%), alpha-humulene (0.57%), cis-farnesol (0.53%), 4-terpineol (0.52%), aciphyllene (0.52%), trans-caryophyllene (0.37%), geranyl acetate (0.36%), cis-2,6-dimethyl-2,6-octadiene (0.34%), octadecane (0.34%), (-)-alpha-terpineol (0.29%), rose oxide trans (0.20%), heneicosane (0.20%), Y-gurjunene (0.19%), 8-heptadecene (0.19%), (9Z)-tricosene (0.19%), dl-limonene (0.17%), beta-bourbonene (0.16%), 3-eicosene, (E)-(0.16%), 1-nonadecene (0.15%), eugenol (0.14%), alloaromadendrene (0.14%), pentadecane (0.12%), 1-nonadecene (0.12%), alphPinene (0.11%), 10-heneicosene (c,t) (0.11%), and rose oxide (0.08%). The results showed that 31.91% of R. damascena consisted of beta-citronellol. Chemical compositions of R. damascena reported from different regions of the world entail citronellol, geraniol, nerol, phenylethyl alcohol, nonadecane, nonadecene, eicosane, heneicosane, tricosane, alpha-guaiene, geranyl acetate, and eugenol [6].
Citronellol is a monoterpenoid with the molecular formula of C10H20O. This chemical can prevent the activities of Staphylococcus aureus and Salmonella typhi. It also possesses a potent inhibitory effect on Candida albicans. Treatment with citronellol in patients receiving radiotherapy and/or chemotherapy has been demonstrated to mitigate the adverse effects of therapy (e.g., dysgeusia, nausea, hearing loss, and numbness of the extremities) and lessens the depletion of leukocytes and neutrophils to ameliorate their immune function [31]. Roses have been shown to have effective healing properties owing to their abundance of beneficial components, fragrant compounds (EOs such as monoterpenes and sesquiterpenes), hydrolysable and condensed tannins, and secondary metabolites such as flavonoids (flavonols, flavones, and anthocyanins). Rose EOs and extracts have therapeutic attributes, including respiratory antiseptics, anti-inflammatories, antioxidants, expectorants, mucolytics, and decongestants. They also can act as symptomatic prophylactics and drugs, thereby alleviating dramatic suffering during severe diseases [32].
Particle Size Distribution and ZP
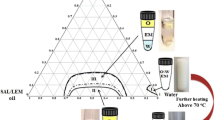
Data and research on the delivery of RDEO using NLCs in the literature are very limited. In this study, RDEO-NLC using the ultrasonication method is reported. The lipid matrix of NLC contains a mixture of solid and liquid lipids (oil), which decreases the melting point of the solid lipid [33]. GMS and SA were employed as solid lipids and OA (liquid oil). In the development of NLC, surfactants reduce the interfacial tension between the lipid and the aqueous phase and, therefore, contribute to the stability of the resultant formulation [33]. In nanoparticles, the selection of a surfactant mixture is performed considering the hydrophilic-lipophilic balance (HLB) of the lipids constituting the nanoparticle matrix and their concentration in the lipid phase of the dispersion [33]. Tn80, Sn60, and Sn80 were the three surfactants selected in this study. Stable nanoemulsions are formed when the aqueous phase, oil phase, HLB, and surfactant concentration are fully matched in the right sequences [34]. As represented in Table 1, four formulations with different amounts of starting materials were prepared to optimize the formulation. Among the four formulations, RDEO-NLC2 was selected as the optimized formulation due to having a smaller particle size with an acceptable PDI and zeta potential. The optimal formulation (RDEO-NLC2) consists of 0.1 g RDEO and OA (liquid lipid, 0.03 g), as well as Sn80 (0.1 g) and Tn80 (0.2 g) as surfactants. The average particle size of the optimal formulation was 78.39 ± 1.52 nm, and its polydispersity index (PDI) and ZP were 0.28 ± 0.01 and − 31.0 mV, respectively (Table 1). The RDEO-NLC2 exhibited an encapsulation efficiency of 99.6% and a loading capacity of 9.6%, with a higher than ZP (± 30 mV), which indicates that the obtained nanoparticle should be stable [35] (Fig. 1).
TEM Study
TEM is one of the most suitable tools for evaluating and analyzing the morphology of nanostructured particles. The morphology of the optimized formulation (RDEO-NLC2) was determined and depicted in Fig. 2A. The results demonstrated a relatively spherical shape for RDEO-NLC2. The relevant histograms (frequency v size) attained by statistical analysis of ~ 500 particle size distributions of RDEO-NLC2 particles were observed to be smaller than 270 nm with a mean particle size of 81.98 ± 49.64 nm (Fig. 2B).
MTT Assay
The MTT method was utilized to check the cell viability and the toxicity effect of cisplatin, RDEO, placebo, and NLC-loaded with RDEO-NLC2 on the MDA-MB-231 BC cell line at 24 and 48 h. The MDA-MB-231 cells were incubated with RDEO, RDEO-NLC2, and placebo at 6.25, 12.5, 25, 50, and 100 ppb concentrations and also with cisplatin in 1, 5, 10, 20, and 50 μg/ml concentrations at 37 °C for 24 and 48 h (Fig. 3). In this study, the conventional chemotherapy drug cisplatin, which contains platinum, was used as a model anticancer drug to be compared to RDEO-NLC2 in terms of cell viability. Cisplatin has undesirable side effects, including allergic reactions, drug resistance, severe kidney problems, decreased immunity to infections, gastrointestinal disorders, hemorrhage, and hearing loss, especially in younger patients [36]. Considering the common side effects of cisplatin, NLC-RDEO2 was formulated to reduce these side effects and to replace cisplatin. In the present study, untreated cells were considered as control. The results presented in Fig. 3 revealed that the RDEO-treated MDA-MB-231 cells did not indicate any toxicity and significant adverse effects on cell proliferation, even at a 100 ppb concentration at 24 and 48 h. After treating the cells with RDEO-NLC2 and cisplatin, cell toxicity was dramatically increased. Based on the MTT assay, cisplatin, followed by RDEO-NLC2, was explored to be more cytotoxic to MDA-MB-231 BC cells after 48 h (Fig. 3). The results also showed that the effect of both cisplatin and RDEO-NLC2 on the MDA-MB-231 cells was dose-dependent (p < 0.001). By increasing the dose of cisplatin to 50 μg/ml and RDEO-NLC2 to 100 ppb, significant effects were found on cell proliferation, and cell survival decreased at 24 and 48 h. The cell viability, however, declined to 38.88 ± 8.63% and 25.33 ± 1.4% (p < 0.001 for both) after exposure to 50 mg/ml of cisplatin after 24 h and 48 h, respectively (Fig. 3). Also, the cell treatment with RDEO-NLC2 complex significantly lessened the viability of the cell to 52.74 ± 1.34% and 39.23 ± 4.92% (p < 0.001 for both) in 100 ppb concentration after 24 h and 48 h, respectively. Based on the results obtained, the cisplatin drug had more negative effects on cell proliferation and higher toxicity than RDEO-NLC2 and placebo at 24 and 48 h. According to the present study and previously published data, nanostructures containing plant EOs with cytotoxic effects are very favorable in drug delivery and cancer control and treatment. Advances in this field reduce the many side effects of cancer treatment. In a previous survey, Kryeziu et al. concluded that nanoencapsulation of Origanum vulgare L. EO into liposomes could contribute to the preservation and improvement of its antioxidant and cytotoxic activity, as well as produce nanocarriers for the development of anticancer agents [37].
In a separate investigation, researchers explored the potential of nanoemulsions containing Mentha piperita essential oil (MPEO) [38]. This essential oil’s anticancer properties were assessed using three distinct human breast cancer cell lines, namely, MCF-7, MDA-MB-231, and MDA-MB-468. The study sought to determine the anticancer effects of MPEO on all of these subtypes of human breast cancer cell lines. Remarkably, the study revealed that the anticancer impact of MPEO achieved within a 24-h treatment using the newly developed nanoemulsions was significantly better than the essential oil with an exposure time of 72 h. The nanoemulsions exhibited excellent cytotoxicity against all the cell lines across all incubation timeframes. These findings hold promise for the potential use of MPEO-loaded nanoemulsions as a novel and effective approach for combating various subtypes of human breast cancer cells [38].
Apoptosis Assay
After incubating the cell line MDA-MB-231 with the IC50 concentration of RDEO-NLC2, cisplatin, and placebo, compared to the control group, at 24 and 48 h, the value of both apoptotic and necrotic cells was determined by flow cytometry. As illustrated in Fig. 4, the percentages of viable (Q4), early (Q3) and late (Q2) apoptotic, and necrotic (Q1) cells in treated cells were different from those in untreated cells. The percentage of viable cells suggested a significant (p < 0.001) decline in the cell viability from 90.53 ± 0.23 and 88.43 ± 2.61 in control cells to 75.00 ± 1.63 and 66.83 ± 1.96 in RDEO-NLC2-treated cells and 62.13 ± 5.08 and 57.03 ± 2.57 in cisplatin-treated cells at 24 and 48 h respectively. According to the results, most of the MDA-MB-231 cells treated with cisplatin and RDEO-NLC2 in the early apoptotic stage at 24 h could remarkably prevent the proliferation of the cancer cells, while at 48 h, the effect of RDEO-NLC2 on these cells was greater than cisplatin. Moreover, cancer cells have evolved complicated mechanisms to antagonize necroptosis and apoptosis. Hence, triggering a single type of programmed cell death may be insufficient for treating cancer metastasis. The selection of varying inducers of cell death or the combined use of various pathway inducers of cell death can help overcome drug resistance to eliminate metastatic cells [39] (Fig. 5).
Apoptosis assay of MDA-MB-231 RDEO-NLC2 IC50-treated cells (A), cisplatin IC50-treated cells (B), placebo IC50-treated cells (C), and control cells (D) for 24 h. The quantitative analysis was plotted to show the population of VC (viable cells), EA (early apoptotic cells), LA (late apoptotic cells), and NC (necrotic cells) cells in 24 h (E)
Apoptosis assay of MDA-MB-231 RDEO-NLC2 IC50-treated cells (A), cisplatin IC50-treated cells (B), placebo IC50-treated cells (C), and control cells (D) for 48 h. The quantitative analysis was plotted to show the population of VC (viable cells), EA (early apoptotic cells), LA (late apoptotic cells), and NC (necrotic cells) cells in 48 h (E)
There is a limited number of studies exploring the impact of Rosa damascena essential oil–loaded nanostructured lipid carriers on breast cancer. However, it is interesting to note a relevant study that focused on the effects of Rosa damascena Mill on colorectal cancer [40]. In this particular research, the findings indicated that R. damascena callus, induced by L-ascorbic acid, exhibited the potential to enhance both growth and secondary metabolite contents. Moreover, it demonstrated significant anti-proliferative, anti-clonogenic, and anti-migratory effects on Caco-2 cancer cells, suggesting its potential as an adjunctive therapy in the context of cancer treatment. This study sheds light on the broader applications of Rosa damascena in the realm of cancer research, potentially extending to breast cancer as well. Further exploration of its efficacy in treating breast cancer through nanostructured lipid carriers remains a promising area for future investigation.
Conclusion
In the present study, RDEO-NLC2 was manufactured effectively using a probe ultrasonication technique. The results confirmed that the developed nanoformulation could boost the efficacy of the RDEO in an NLC preparation. Cisplatin induced more cytotoxic properties than RDEO-NLC2 against MDA-MB-231 BC cells. However, according to the result achieved, RDEO-NLC2 could serve as a therapeutic agent for the treatment of BC. As cisplatin has serious side effects, and the current study suggests that RDEO-NLC2 has anticancer effects in vitro, further investigations are necessary in vivo to affirm its anticancer effect.
Data Availability
Data is available upon request.
References
Ashtari A, Niazvand F, Khorsandi L. Chemotherapy drugs based on solid lipid nanoparticles for breast cancer treatment. Medicina (Kaunas). 2020. https://doi.org/10.3390/medicina56120694.
Waks AG, Winer EP. Breast cancer treatment. JAMA. 2019. https://doi.org/10.1001/jama.2018.19323.
Hazafa A, Rehman KU, Jahan N, Jabeen Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutrition and Cancer. 2020. https://doi.org/10.1080/01635581.2019.1637006.
Carqueijeiro I, Langley C, Grzech D, Koudounas K, Papon N, O’Connor SE, Courdavault, V. Beyond the semi-synthetic artemisinin: metabolic engineering of plant-derived anti-cancer drugs. Curr Opin Biotechnol. 2020. https://doi.org/10.1016/j.copbio.2019.11.017.
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020. https://doi.org/10.1016/j.plaphy.2020.01.006.
Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med. 2015. https://doi.org/10.1016/j.jtcme.2015.09.005.
Lee S, Park YR, Kim SH, Park EJ, Kang MJ, So I, Chun JN, Jeon JH. Geraniol suppresses prostate cancer growth through down‐regulation of E2F8. Cancer Med. 2016. https://doi.org/10.1002/cam4.864.
Ho Y, Suphrom N, Daowtak K, Potup P, Thongsri Y, Usuwanthim K (2020) Anticancer effect of citrus hystrix DC. Leaf extract and its bioactive constituents citronellol and, citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceuticals (Basal). 2020. https://doi.org/10.3390/ph13120476.
Cebi, N. Quantification of the geranium essential oil, palmarosa essential oil and phenylethyl alcohol in Rosa damascena essential oil using ATR-FTIR spectroscopy combined with chemometrics. Foods. 2021. https://doi.org/10.3390/foods10081848.
Akram M, Riaz M, Munir N, Akhter N, Zafar S, Jabeen F, Shariati MA, Akhtar N, Riaz Z, Altaf SH, Daniyal M, Zahid R, Said Khan F. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: a literature review. J Pharm Pharmacol. 2020. https://doi.org/10.1111/jphp.13185.
Al-Oqail M, Farshori NN, Al-Sheddi ES, Al-Massarani SM, Saquib Q, Siddiqui MA, Al-Khedhairy AA. Oxidative stress mediated cytotoxicity, cell cycle arrest, and apoptosis induced by Rosa damascena in human cervical cancer HeLa cells. Oxid Med Cell Longev. 2021. https://doi.org/10.1155/2021/6695634.
Irshad M, Subhani MA, Saqib A, Hussain A. Biological importance of essential oils. https://doi.org/10.5772/intechopen.77673.
Saporito F, Sandri G, Bonferoni MC, Rossi S, Boselli C, Icaro Cornaglia A, Mannucci B, Grisoli P, Vigani B, Ferrari F. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomedicine. 2017. https://doi.org/10.2147/IJN.S152529.
Mele E. Electrospinning of essential oils. Polymers. 2020. https://doi.org/10.3390/polym12040908.
Elbagory AM, Marima RM, Dlamini Z. Role and merits of green based nanocarriers in cancer treatment. Cancers. 2021. https://doi.org/10.3390/cancers13225686.
Ahmad J, Rizwanullah M, Amin S, Warsi MH, Ahmad MZ, Barkat, MA. A nanostructured lipid carriers (NLCs): nose-to-brain delivery and theranostic application. Curr Drug Metab. 2020. https://doi.org/10.2174/1389200221666200719003304.
Rizwanullah, M, Ahmad MZ, Garg A, Ahmad J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim Biophys Acta Gen Subj. 2021. https://doi.org/10.1016/j.bbagen.2021.129936.
Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019. https://doi.org/10.1016/j.bioorg.2019.102925.
Wei G, Wang Y, Yang G, Wang Y, Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021. https://doi.org/10.7150/thno.57828.
Bose S, Du Y, Takhistov P, Michniak-Kohn B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int J Pharm. 2013. https://doi.org/10.1016/j.ijpharm.2012.12.013.
Najjari N, Sari S, Saffari M, Kelidari H, Nokhodchi A. Formulation optimization and characterization of Pistacia atlantica Desf. essential oil-loaded nanostructured lipid carriers on the proliferation of human breast cancer cell line SKBR3 (in vitro studies). J Herb Med. 2022. https://doi.org/10.1016/j.hermed.2022.100600.
Mondéjar-López M, López-Jiménez AJ, Martínez JCG, Ahrazem O, Gómez-Gómez L, Niza E. Thymoquinone-loaded chitosan nanoparticles as natural preservative agent in cosmetic products. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23020898.
Yazdani Ashtiani, S, Ahmad Nasrollahi S, Naeimifar A, Nassiri Kashani, A., Samadi, A., Yadangi, S., Aboutaleb E, Abdolmaleki P, Dinarvand R, Firooz A. Preparation and safety evaluation of topical simvastatin loaded NLCs for vitiligo. Adv Pharm Bull. https://doi.org/10.34172/apb.2021.011.
Huang Z, Yu P, Tang J. Characterization of triple-negative breast cancer MDA-MB-231 cell spheroid model. Onco Targets Ther. 2020. https://doi.org/10.2147/ott.s249756..
Izham M N M , Hussin Y, Rahim N F C, Aziz M N M, Yeap S K, Rahman H S, Masarudin, M J, Mohamad, N E. Abdullah, R, & Alitheen N B. Physicochemical characterization, cytotoxic effect and toxicity evaluation of nanostructured lipid carrier loaded with eucalyptol. BMC Complement Altern Med. 2021. https://doi.org/10.1186/s12906-021-03422-y.
Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harbor Protocols. 2018. https://doi.org/10.1101/pdb.prot095505.
Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006. https://doi.org/10.1038/nprot.2006.238.
Telford WG. Multiparametric analysis of apoptosis by flow cytometry. Methods Mol Biol. 2018. https://doi.org/10.1007/978-1-4939-7346-0_10.
Liu Y, Yang G, Jin S, Xu L, Zhao C. Development of high‐drug‐loading nanoparticles. ChemPlusChem. 2020. https://doi.org/10.1002/cplu.202000496.
Zhao M, van Straten D, Broekman MLD, Préat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020. https://doi.org/10.7150/thno.38147.
Yu WN, Lai YJ, Ma JW, Ho CT, Hung SW, Chen YH, Chen CT, Kao JY, Way TD. Citronellol induces necroptosis of human lung cancer cells via TNF-α pathway and reactive oxygen species accumulation. in vivo. 2019. https://doi.org/10.21873/invivo.11590.
Mileva M, Ilieva Y, Jovtchev G, Gateva S, Zaharieva MM, Georgieva A, Dimitrova, Dobreva A, Angelova T, Vilhelmova-Ilieva N, Valcheva V, Najdenski H. Rose flowers—a delicate perfume or a natural healer? Biomolecules. 2021. https://doi.org/10.3390/biom11010127.
Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, Mahant S. SLN and NLC for topical, dermal, and transdermal drug delivery. Exp Opin Drug Deliv. 2020. https://doi.org/10.1080/17425247.2020.1727883.
Park YH, Kim HJ. Formulation and stability of horse oil-in-water emulsion by HLB system. Food Sci Biotechnol. 2021. https://doi.org/10.1007/s10068-021-00934-8.
Mohamed MI, Abdelbary AA, Kandil SM, Mahmoud TM. Preparation and evaluation of optimized zolmitriptan niosomal emulgel. Drug Dev Ind Pharm. 2019. https://doi.org/10.1080/03639045.2019.
Dasari S, Bernard Tchounwou P. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014. https://doi.org/10.1016/j.ejphar.2014.07.025.
Kryeziu TL, Haloci E, Loshaj-Shala A, Bagci U, Oral A, Stefkov GJ, Zimmer A, Basholli-Salihu M. Nanoencapsulation of Origanum vulgare essential oil into liposomes with anticancer potential. Pharmazie. 2022. https://doi.org/10.1691/ph.2022.1230.
Abedinpour N, Ghanbariasad A, Taghinezhad A, & Osanloo M. Preparation of nanoemulsions of Mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience. 2021. https://doi.org/10.1007/s12668-021-00827-4.
Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015. https://doi.org/10.1186/s12943-015-0321-5.
Darwish H, Alharthi S, Mehanna RA, Ibrahim SS, Fawzy MA, Alotaibi SS, Albogami SM, Albogami B, Hassan SH, Noureldeen A. Evaluation of the anti-cancer potential of Rosa damascena Mill. Callus extracts against the human colorectal adenocarcinoma cell line. In Molecules . 2022. https://doi.org/10.3390/molecules27196241.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by EY, SS, HK, KA-A, and AN. The first draft of the manuscript was written by EY, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: SS and HK. Methodology: SS. Formal analysis and investigation: EY. Writing—original draft preparation: EY. Writing—review and editing: SS, KA-A, and AN. Funding acquisition: EY. Supervision: SS and AN.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yari, E., Sari, S., Kelidari, H. et al. Effect of Rosa damascena Essential Oil Loaded in Nanostructured Lipid Carriers on the Proliferation of Human Breast Cancer Cell Line MDA-MB-231 in Comparison with Cisplatin. J Pharm Innov 19, 4 (2024). https://doi.org/10.1007/s12247-024-09809-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12247-024-09809-x