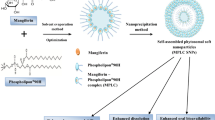
Abstract
Purpose
Andrographis paniculata is a promising extract that has gained attention due to its broad range of pharmacological effects. It is now being researched extensively as a natural hepatoprotective surrogate with potential efficacy against alcohol-induced hepatotoxicity. However, it has several flaws that restrict its therapeutic value, including less bioavailability due to inadequate lipophilic solubility and indigent gastrointestinal absorption. This study highlights the development of self-assembled phytosome nanocarriers to improve lipophilic solubility and bioavailability. In this study, nanophytosomes of Andrographis paniculata extract (APE) were engineered to improve the rate of drug release and hepatoprotective efficiency of the extract.
Method
The nanophytosomes of Andrographis paniculata (APP) were formulated utilizing a full factorial design technique, which took into account a variety of variables that could lead to an optimized formulation. Particle size analysis, zeta potential, differential scanning calorimetry (DSC), Fourier transformation infrared spectroscopy (FTIR), powder X-ray diffractometer (PXRD), proton nuclear magnetic resonance (1H NMR), scanning electron microscopy (SEM), and solubility studies were used for physicochemical characterization.
Result
The decrease in the crystalline nature of nanophytosome was shown by PXRD and SEM. The production of the phyto-phospholipid complex was verified with the help of 1H NMR, DSC, and FTIR. The dissolution rate in nanophytosomes was also observed to be increased and sustained. Furthermore, in pharmacodynamics assessment, APP was found more effective as compared to APE.
Conclusion
Overall, the findings of this study revealed that nanophytosome formulation might be used as promising drug delivery for sustained drug release and improved hepatoprotection efficacy.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- APE:
-
Andrographis paniculata Extract
- APP:
-
Andrographis paniculata Nanophytosomes
- FTIR:
-
Fourier transformation infrared spectroscopy
- DSC:
-
Differential scanning calorimetry
- 1H NMR:
-
Proton nuclear magnetic resonance
- PXRD:
-
Powder X-ray diffractometer
- SEM:
-
Scanning electron microscopy
- SPC:
-
Soya phosphatidylcholine
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Aspartate aminotransferase
- γ-GT:
-
Gamma-glutamyl transferase
- TB:
-
Total bilirubin
- TP:
-
Total protein
- LDH:
-
Lactate dehydrogenase
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
References
El-Newary SA, Shaffie NM, Omer E. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac J Trop Med. 2017;10(4):361–71.
Abou Seif HS. Ameliorative effect of pumpkin oil (Cucurbita pepo L.) against alcohol-induced hepatotoxicity and oxidative stress in albino rats. Beni-Suef Univ J Basic Appl Sci. 2014;3(3):178–85.
Clemens DL, Jerrells TR. Ethanol consumption potentiates viral pancreatitis and may inhibit pancreas regeneration: preliminary findings. Alcohol. 2004;33(3):183–9.
Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124(3):778–90.
Ramudu SK, Korivi M, Kesireddy N, Chen C-Y, Kuo CH, Kesireddy SR. Ginger feeding protects against renal oxidative damage caused by alcohol consumption in rats. J Ren Nutr. 2011;21(3):263–70.
Baskaran M, Periyasamy L, Rajagopalan R. Effect of Phyllanthus niruri on alcohol and polyunsaturated fatty acid induced oxidative stress in liver. Int J Pharm Pharm Sci. 2010;2(4):58–62.
Habib-ur-Rehman M, Mahmood T, Salim T, Afzal N, Ali N, Iqbal J, et al. Effect of silymarin on serum levels of ALT and GGT in ethanol induced hepatotoxicity in albino rats. J Ayub Med Coll Abbottabad. 2009;21(4):73–5.
Nrabalu CC. The effects of alcohol administration on serum lipid profile, total protein and liver enzymes in rats: University Of Nigeria Nsukka. 2011.
Petri W. Antimicrobial Agents In: Hardman JG, Limbird LE. Eds. Goodman and Gillman’s: the pharmacological basis of therapeutics. New York: Mc Graw Hill Companies Inc. 2001.
Handa S, Sharma A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J Med Res. 1990;92:276–83.
Saraswat B, Visen PS, Patnaik G, Dhawan B. Effect of andrographolide against galactosamine-induced hepatotoxicity. Fitoterapia (Milano). 1995;66(5):415–20.
Zhang C, Kuroyangi M, Tan BK. Cardiovascular activity of 14-deoxy-11, 12-didehydroandrographolide in the anaesthetised rat and isolated right atria. Pharmacol Res. 1998;38(6):413–7.
Kapil A, Koul I, Banerjee S, Gupta B. Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem Pharmacol. 1993;46(1):182–5.
Verma VK, Sarwa KK, Kumar A, Zaman MK. Comparison of hepatoprotective activity of Swertia chirayita and Andrographis paniculata plant of North–East India against CCl4 induced hepatotoxic rats. J Pharm Res. 2013;7(7):647–53.
Syukri Y, Martien R, Lukitaningsih E, Nugroho AE. Quantification of andrographolide isolated from Andrographis paniculata Nees obtained from traditional market in Yogyakarta using validated HPLC. Indones J Chem. 2016;16(2):190–7.
Panossian A, Hovhannisyan A, Mamikonyan G, Abrahamian H, Hambardzumyan E, Gabrielian E, et al. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7(5):351–64.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10.
Jog R, Burgess DJ. Pharmaceutical amorphous nanoparticles. J Pharm Sci. 2017;106(1):39–65.
Müllertz A, Ogbonna A, Ren S, Rades T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J Pharm Pharmacol. 2010;62(11):1622–36.
Taupitz T, Dressman JB, Buchanan CM, Klein S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: itraconazole. Eur J Pharm Biopharm. 2013;83(3):378–87.
Syukri Y, Fernenda L, Utami FR, Qiftayati I, Kusuma AP, Istikaharah R. Preperation and characterization of Β-cyclodextrin inclusion complexes oral tablets containing poorly water soluble glimipiride using freeze drying method. Indones J Pharm. 2015;26(2):71.
Ramadhani N, Shabir M, McConville C. Preparation and characterisation of Kolliphor® P 188 and P 237 solid dispersion oral tablets containing the poorly water soluble drug disulfiram. Int J Pharm. 2014;475(1–2):514–22.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems–an overview. Acta Pharmaceutica Sinica B. 2013;3(6):361–72.
Syukri Y, Martien R, Lukitaningsih E, Nugroho AE. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: characterization, in-vitro and in-vivo assessment. J Drug Deliv Sci Technol. 2018;47:514–20.
Lu M, Qiu Q, Luo X, Liu X, Sun J, Wang C, et al. Phyto-phospholipid complexes (nanophytosomes): a novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci. 2019;14(3):265–74.
Semalty A. Cyclodextrin and phospholipid complexation in solubility and dissolution enhancement: a critical and meta-analysis. Expert Opin Drug Deliv. 2014;11(8):1255–72.
Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev. 1994;52(10):327–39.
Lieber CS, Decarli LM, Mak KM, Kim CI, Leo MA. Attenuation of alcohol-induced hepatic fibrosis by polyunsaturated lecithin. Hepatology. 1990;12(6):1390–8.
Singh A, Kumar K, Gupta A, Singh S. Nanophytosomes: a newer approach towards drug delivery system. World J Pharm Res. 2019:39–43.
Mazumder A, Dwivedi A, Du Preez JL, Du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin–phytosome complex. Int J Pharm. 2016;498(1–2):283–93.
Telange DR, Patil AT, Pethe AM, Fegade H, Anand S, Dave VS. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur J Pharm Sci. 2017;108:36–49.
Saoji SD, Raut NA, Dhore PW, Borkar CD, Popielarczyk M, Dave VS. Preparation and evaluation of phospholipid-based complex of standardized centella extract (SCE) for the enhanced delivery of phytoconstituents. AAPS J. 2016;18(1):102–14.
Maiti K, Mukherjee K, Murugan V, Saha BP, Mukherjee PK. Exploring the effect of hesperetin–HSPC complex—a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10(3):943–50.
Hekmatpanah J, Haghighat N, Adams CR. Alcohol consumption by nursing rats and its effect on the cerebellum of the offspring. Alcohol and Alcoholism. 1994;29(5):535–47.
Ohkawa H, Ohishi W, Yagi K. Colorimetric method for determination of MDA activity. Biochemistry. 1979;95:351.
Hugo A. Catalase. Methods of enzymatic analysis, 2nd ed UB Hans (ed) Academic Press, New Yolk. 1974:673–4.
Sun S, Yang S, Dai M, Jia X, Wang Q, Zhang Z, et al. The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose-stimulated H9C2 cells. BMC Complement Altern Med. 2017;17(1):1–12.
Nandi A, Chatterjee I. Assay of superoxide dismutase activity in animal tissues. J Biosci. 1988;13(3):305–15.
LeFevre M, Olivo R, Vanderhoff J, Joel D. Accumulation of latex in Peyer’s patches and its subsequent appearance in villi and mesenteric lymph nodes. Proc Soc Exp Biol Med. 1978;159(2):298–302.
Savić R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300(5619):615–8.
Hou Z, Li Y, Huang Y, Zhou C, Lin J, Wang Y, et al. Nanophytosomes loaded with mitomycin C–soybean phosphatidylcholine complex developed for drug delivery. Mol Pharm. 2013;10(1):90–101.
Yu F, Li Y, Chen Q, He Y, Wang H, Yang L, et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based nanophytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur J Pharm Biopharm. 2016;103:136–48.
Yi H, Yang Z, Yongfang Z. The purification and the identification of lecithin and its application. Amino Acids and Biotic Resources. 2001;23(2):28–31.
Cai X, Luan Y, Jiang Y, Song A, Shao W, Li Z, et al. Huperzine A–phospholipid complex-loaded biodegradable thermosensitive polymer gel for controlled drug release. Int J Pharm. 2012;433(1–2):102–11.
Shivanand P, Kinjal P. Nanophytosomes: technical revolution in phytomedicine. Int J Pharmtech Res. 2010;2(1):627–31.
Jena SK, Singh C, Dora CP, Suresh S. Development of tamoxifen-phospholipid complex: novel approach for improving solubility and bioavailability. Int J Pharm. 2014;473(1–2):1–9.
Udapurkar PP, Bhusnure OG, Kamble SR. Diosmin nanophytosomes: development, optimization and physicochemical characterization. Indian J Pharm Educ Res. 2018;52(4):S29–36.
Freag MS, Saleh WM, Abdallah OY. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int J Pharm. 2018;535(1–2):18–26.
Maryana W, Rachmawati H, Mudhakir D. Formation of phytosome containing silymarin using thin layer-hydration technique aimed for oral delivery. Materials today: Proceedings. 2016;3(3):855–66.
Lasonder E, Weringa WD. An NMR and DSC study of the interaction of phospholipid vesicles with some anti-inflammatory agents. J Colloid Interface Sci. 1990;139(2):469–78.
Venema FR, Weringa WD. The interactions of phospholipid vesicles with some anti-inflammatory agents. J Colloid Interface Sci. 1988;125(2):484–92.
Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 2006;307(1):77–82.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Begum MM, Kiran KR. Evaluation of methanolic extract of Cleome chelidonii for hepatoprotective activity against paracetamol and ethanol induced hepatotoxicity in rats. Int J Pharm Sci Rev Res. 2016;5(1):28–36.
Karwani G, Sisodia S. Hepatoprotective activity of Tagetes erecta Linn. in ethanol induced hepatotoxicity in rats. Sch Acad J Pharm. 2015;4(3):181–9.
Epstein M. Renal sodium handling in liver disease. The kidney in liver disease. 1988:3–30.
Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? The lancet. 1994;344(8924):721–4.
Maruthappan V, Shree KS. Hepatoprotective effect of Azadirachta indica (Neem) leaves against alcohol induced liver injury in Albino rats. J Pharm Res. 2009;2(4):655–9.
Kharpate S, Vadnerkar G, Jain D, Jain S. Evaluation of hepatoprotective activity of ethanol extract of Ptrospermum acerifolium ster leaves. Indian J Pharm Sci. 2007;69(6):850.
Mukherjee K. Quality control of herbal drugs. New Business Horrizons. New Delhi: Pharmaceutical Publishers. 2002.
Acknowledgements
The authors are also thankful to Amsar Goa Pvt. Ltd, Goa, India for providing Andrographispaniculata extract and VAV Pvt. Ltd, Mumbai, India, for providing Soya Phosphatidylcholine (SPC; LECIVA-S70) as gift samples. The authors also extend thanks to Shivaji University, Kolhapur, India to perform characterization studies. The authors also extend gratitude to Crystal Biology Solutions, Pune, India for carrying out in vivo activity. The laboratory facilities provided by Principal, BharatiVidyapeeth College of Pharmacy, Kolhapur, India and Principal, Indira College of Pharmacy, Pune, India are also acknowledged gratefully.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karekar, P., Killedar, S. & More, H. Nanophytosomes Loading Andrographis paniculata Hydroalcoholic Extract: Promising Drug Delivery for Hepatoprotective Efficacy. J Pharm Innov 18, 1084–1099 (2023). https://doi.org/10.1007/s12247-023-09712-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09712-x