Abstract
The paradigm shift in the pharmaceutical industry to continuous manufacturing, which has recently progressed from conceptual demonstration to pilot production, has stimulated the development and application of process systems engineering (PSE) tools for implementing efficient and robust control strategies. In this study, a systematic framework for process control design and risk analysis for continuous pharmaceutical solid-dosage manufacturing is proposed, consisting of system identification with state-space models; control design and analysis metrics; hierarchical three-layer control structures; risk mapping, assessment and planning (Risk MAP) strategies; and control performance indicators. The framework is applied to a feeding-blending system, wherein the major source of variance in the product quality arises. It can be demonstrated that the variance in the feeding-blending system can be mitigated and managed through the proposed systematic framework for control design and risk analysis. The process analytical technology (PAT) tool for mass fraction measurement of active pharmaceutical ingredient (API) and its relative standard deviation (RSD) were indispensable to achieve an efficient control design at the advanced layers. Specifically, the improvements in control performance by implementing advanced model-based control strategy are found to be limited by model-plant mismatch and the sampling time of the PAT tools.













Similar content being viewed by others
References
Ierapetritou M, Muzzio F, Reklaitis G. Perspectives on the continuous manufacturing of powder-based pharmaceutical processes. AICHE J. 2016;62(6):1846–62.
Lee SL, O'Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov. 2015;10(3):191–9.
Coupe A. P2, or not P2: that is the question from development to design, in CMAC Annual Open Day, Glasgow, United Kingdom, 2015.
Singh R, Ierapetritou M, Ramachandran R. An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction. Int J Pharm. 2012;438:307–26.
Singh R, Muzzio FJ, Ierapetritou M, Rohit R. A combined feed-forward/feed-back control system for a QbD-based continuous tablet manufacturing process. PRO. 2015;3:339–56.
Singh R, Sahay A, Muzzio F, Ierapetritou M, Rohit R. A systematic framework for onsite design and implementation of a control system in a continuous tablet manufacturing process. Comput Chem Eng. 2014;66:186–200.
Lakerveld R, Benyahia B, Braatz RD, Barton PI. Model-based design of a plant-wide control strategy for a continuous pharmaceutical plant. AICHE J. 2013;59(10):3671–85.
Singh R, Gernaey KV, Gani R. Model-based computer-aided framework for design of process monitoring and analysis systems. Comput Chem Eng. 2009;33(1):22–42.
Singh R, Sen M, Ierapetritou M, Ramachandra R. Integrated moving horizon-based dynamic real-time optimization and hybrid MPC-PID control of a direct compaction continuous tablet manufacturing process. J Pharm Innov. 2015;10:233–53.
Ramachandran R, Arjunan J, Chaudhury A, Ierapetritou MG. Model-based control-loop performance of a continuous direct compaction process. J Pharm Innov. 2011;6:249–63.
Lakerveld R, Benyahia B, Heider PL, Zhang H, Wolfe A, Testa CJ, et al. The application of an automated control strategy for an integrated continuous pharmaceutical pilot plant. Org Process Res Dev. 2015;19:1088–100.
Kourti T, Davis B. The business benefits of quality bu design (QbD). Pharm Eng. 2012;32(4):1–10.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding pharmaceutical quality by design. AAPS J. 2014;16(4):771–83.
Gupta A, Giridhar A, Venkatasubramanian V, Reklaitis GV. Intelligent alarm management applied to continuous pharmaceutical tablet manufacturing: an integrated approach. Ind Eng Chem Res. 2013;52:12357–68.
Darby ML, Nikolaou M. MPC: current practice and challenges. Control Eng Pract. 2012;20:328–42.
Pernenkil L. Continuous blending of dry pharmaceutical powders. Boston, MA: Massachusetts Institute of Technology; 2008.
U. FDA, U.S. Guidance for Industry: Q8(2) Pharmaceutical Development Maryland, Food and Drug Administration, 2009.
Zhao XJ, Gatumel C, Dirion JL, Berthiaux H, Cabassud M. Implementation of a control loop for a continuous powder mixing process. In: Proceeding of the 2013 AIChE Annual Meeting. San Francisco; 2013.
Previdi F, Belloli D, Cologni A, Savaresi SM, Cazzola D, Madaschi M. Control system design for a continuous gravimetric blender. In: Preprints of the 18th IFAC World Congress, Milano, Italy, 2011.
Rehrl J, Kruisz J, Sacher S, Khinast J, Horn M. Optimized continuous pharmaceutical manufacturing via model-predictive control. Int J Pharm. 2016;510:100–15.
Rogers AJ, Hashemi A, Ierapetritou MG. Modeling of particular processes for the continuous manufacture of solid-based pharmaceutical dosage forms. PRO. 2013;1:67–127.
Su Q, Schiano S, Wu CY, Nagy ZK, Rielly CD. Dynamic impact milling model with a particle-scale breakage kernel. Comput Aided Chem Eng. 2016;38:475–80.
Hsu S-H, Reklaitis GV, Venkatasubramanian V. Modeling and control of roller compaction for pharmaceutical manufacturing. Part I: process dynamics and control framework. J Pharm Innov. 2010;5:14–23.
Hsu S-H, Reklaitis GV, Venkatasubramania V. Modeling and control of roller compaction for pharmaceutical manufacturing Part II: control system design. J Pharm Innov. 2010;5:24–36.
Benyahia B, Lakerveld R, Barton PI. A plant-wide dynamic model of a continuous pharmaceutical process. Ind Eng Chem Res. 2012;51(47):15393–412.
Su Q-L, Hermanto MW, Braatz RD, Chiu M-S. Just-in-time-learning based extended prediction self-adaptive control for batch processes. J Process Control. 2016;43:1–9.
Asmar BN. Control of a two-stage refrigeration system. Nottingham; 1999.
Joseph B, Brosilow CB. Inferential control of processes. AICHE J. 1978;24:485–92.
Morari M. Design of resilient processing plants-III. A general framework for the assessment of dynamic resilience. Chem Eng Sci. 1983;38:1881–91.
Hovd M, Skogestad S. Simple frequency-dependent tools for control system analysis, structure selection and design. Automatica. 1992;28:989–96.
Koung C-W, MacGregor JF. Robustness of multivariable linear controllers to process nonlinearities. Ind Eng Chem Res. 1992;31:1085–96.
Seborg DE, Edgar TF, Mellichamp DA. Process dynamics and control. Hoboken: John Wiley & Sons Inc.; 2004.
Rockoff JD. Drug making breaks away from its old ways, 8 February 2015. [Online]. Available: http://www.wsj.com/articles/drug-making-breaks-away-from-its-old-ways-1423444049. [Accessed 6 Oct 2016].
Vanarase AU, Alcalà M, Jerez Rozo JI, Muzzio FJ, Romañach RJ. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem Eng Sci. 2010;65:5728–33.
Austin J, Gupta A, McDonnell R, Reklaitis GV, Harris MT. A novel microwave sensor to determine particulate blend composition on-line. Anal Chim Acta. 2014;819:82–93.
Ganesh S, Troscinski R, Schmall N, Lim J, Nagy Z, Reklaitis G. Application of x-ray sensors for in-line and non-invasive monitoring of mass flow rate in continuous tablet manufacturing. J Pharm Sci, p. In review. 2017;
Kenney C, Hewer G. Necessary and sufficient conditions for balancing unstable systems. IEEE Trans Autom Control. 1987;32(2):157.
Antoulas AC. Approximation of large-scale dynamical systems. In: Ralph NCSU, Smith C, editors. Philadelphia: Society for Industrial and Applied Mathematics; 2005.
Franklin GF, Powell JD, Workman ML. Digital control of dynamic systems. 2nd ed. Boston: Addison-Wesley; 1990.
Nagy ZK, Mahn B, Franke R, Allgöwer F. Evaluation study of an efficient output feedback nonlinear model predictive control for temperature tracking in an industrial batch reactor. Control Eng Pract. 2007;15:839–50.
Huang J, Pla DL. GMP Implementation of advanced process control in tablet manufacturing, American Pharmaceutical Review, no. March, 2017.
Wang S-H, Zachery R. Singular value decomposition of system input-output matrix and its sysmmetry property. Comput Electr Eng. 1996;22(3):231–4.
Zhu Z-X. Variable pairing selection based on individual and overall interaction measures. Ind Eng Chem Res. 1996;35:4091–9.
Lau H, Alvarez J, Jensen KF. Synthesis of control structures by singular value analysis: dynamic measures of sensitivity and interaction. AICHE J. 1985;31:427–39.
Grosdidier P, Morari M. Interaction measures for systems under decentralized control. Automatica. 1986;22:309–19.
Feng W, Grimble MJ. A new procedure to account for performance interaction in multivariable systems. In: Proceedings of the American Control Conference, Pittsburgh, PA, 1989.
Portillo P, Muzzio F, Ierapetritou M. Using compartment modeling to investigate mixing behavior of a continuous mixer. J Pharm Innov. 2008;3(3):161–74.
Acknowledgements
The authors wish to acknowledge the financial support of the Food and Drug Administration under the grant number DHHS-FDA U01FD005535-01.
Author information
Authors and Affiliations
Corresponding authors
Appendices
Appendix A: Control Design and Analysis Metrics
Singular Value Decomposition (SVD)
The SVD produces a diagonal matrix Σ, which is of the same size of the transfer function matrix G and with nonnegative diagonal elements σ i in decreasing order, and unitary matrices U and V, as shown below.
The set of singular values σ i corresponds to the system gains, if σ i is close or equal to zero, then the input required to produce the output must have very large magnitude, or be infinite [42].
Condition Number(CN)
The CN of the transfer function matrix G is defined as the ratio of the largest singular value of σ i to the smallest,
In general, large values of condition number indicate the process control system is difficult to be decoupled. An ideal system would have a CN number of one.
Morari’s Resilience Index (MRI)
MRI is simply the minimum singular value of σ i of the process transfer function matrix G [29].
Its criterion is that the larger the minimum singular value, the matrix G is further from being singular and hence the more resilient the process is.
Niederlinski Index (NI)
The NI characterizes the stability of a closed-loop systems with integral action, as defined below:
where П is the product of the diagonal elements of G(0), and |G(0)| is the determinant of G(0). The criterion is that if the NI is negative then the closed-loop system is unstable; otherwise, the system may or may not be stable. Note that NI is a necessary but not sufficient condition [27].
Relative gain array (RGA)
The RGA analysis is the most widely used tool in interaction analysis. For a non-singular transfer function matrix G with steady state gains, viz., s = 0, the RGA, denoted by Λ, is defined as:
where the element λ ij in Λ is a qualitative measure of interaction for process input u i and output y j . A control pair with a λ ij value close to 1 is preferred [30].
Relative Interaction Array (RIA)
Unlike the RGA, the RIA represents an improved interaction measure, which is the relative amount of interaction in the loop and it is quantitative, as defined below.
wherein the distance of an RIA element from 0 actually quantifies the amount of interaction in a loop. Hence the design criterion is to pair the control variables with RIA elements as close as possible to 0 [43].
Direct Interaction Measure (DIM)
The DIM measures how far the process as represented by the transfer function matrix G is from being a completely decoupled system [44], by partitioning the unitary matrices of U and V as defined in Eq. (A1) for SVD analysis of G:
where u i and v i are the corresponding vectors in U and V to the ith singular value σ i . The θ values range between 0–90o, with a value of zero indicating no interaction exists, and values above 15o suggesting the need to introduce some compensation to reduce the level of interaction within the system.
μ-Based Interaction Measure (μIM)
In a decentralized control structure, viz., the multi SISO loops at L1, the off-diagonal elements in the process transfer matrix G are ignored, leading to the interaction in the control system and hence the performance degradation, which can be measured by the μIM index [45].
This μIM index quantifies the difference between a plant G and its approximation G d with diagonal elements of G only and guarantees the stability of the full closed loop transfer function matrix H [27]:
where K d is the diagonal control matrix, by applying a bound on the magnitude of the decentralized closed matrix H d:
such as
The matrices E1 and E2 are known as the relative error matrices and are defined as
The μIM is implemented by examining the values of μ(E1) such that if the system G is stable, then the plant will be decentralized integral controllable, i.e., there exists a stabilizing decentralized controller with integral action such that each individual loop may be tuned independently by a factor of 0 to 1 without introducing instability, if
Performance Interaction Measure (PIM)
The PIM measure (0 < τ < 2) describes the interaction of a process control system when the pairing is fixed [46], as also calculated based on the SVD analysis:
wherein a smaller τ indicates lower performance interaction.
Appendix B: Mathematical models
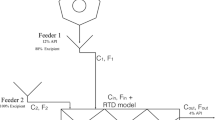
Feeder
The feeder was simulated using a transfer function of first order plus time delay (FOPTD), as shown below.
where k is the process gain, θ is the time delay, and τ is the time constant.
Blender
The blender model consists of 2D compartments extending in the axial and radial directions. Each compartment is equally sized and assumed to be well mixed. The mass balance for a powder component (e.g., API, excipient) in a compartment was represented as below [47].
where m is the mass holdup in the compartment, t is time, and i and j are the indices of the compartment in the axial and radial directions, respectively. F f , F b , and F r are the forward, backward, and radial fluxes, respectively, which are obtained as below:
where ω is the blender speed and the rest are constant parameters and are estimated from experimental data. The API mean composition and its mixing relative standard deviation are calculated based on the API mass fractions in the compartments at the blender exit.
Appendix C: Process and Control Parameters
Rights and permissions
About this article
Cite this article
Su, Q., Moreno, M., Giridhar, A. et al. A Systematic Framework for Process Control Design and Risk Analysis in Continuous Pharmaceutical Solid-Dosage Manufacturing. J Pharm Innov 12, 327–346 (2017). https://doi.org/10.1007/s12247-017-9297-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-017-9297-6