Abstract
Understanding how natural and anthropogenic disturbances affect the structure and functioning of marine ecosystems is central to predicting future dynamics. Placentia Bay is an Ecologically and Biologically Significant Area (EBSA) in the North Atlantic exposed to multiple stressors (e.g., rising sea surface temperatures, tanker traffic, and aquaculture). To investigate changes in the community and functional structure of soft-sediment macrofauna as well as environmental drivers of observed variation, we compared contemporary (2019–2020) and historical (1998) samples at eight stations (n = 77) collected 21 years apart. Although community and functional structure differed between these time points, functional traits were maintained (i.e., no loss of 36 trait modalities). Overall, 37% of species/taxa were only observed in either the historical or contemporary community, and the contemporary community exhibited lower macrofaunal density but had similar richness, resulting in higher evenness and diversity. Highly tolerant subsurface deposit feeders having small body sizes (< 10 mm) and direct development dominated the historical community. The contemporary community had nearly equal proportions of surface and subsurface deposit feeders with small to medium body sizes (< 10–50 mm) with pelagic larvae, and the proportion of highly tolerant species/taxa was reduced. These changes likely reflect the reduction in polychaetes (91 vs. 58%) and increased bivalves (4 vs. 25%) relative to the historical time point. Community variation was driven by changes in the sedimentary habitat. Contemporary versus historical sediments were ~ 4.5x coarser (possibly due to storms) with higher levels of sedimentary organic matter. This work contributes to advancing the understanding of relationships between benthic macrofauna, functional traits, and the sedimentary habitat in coastal environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With global climate change altering ecosystem conditions, information pertaining to both community structure and ecosystem function is necessary to understand and determine important drivers of environmental change and predict the resilience of communities (Biggs et al. 2020; Vause et al. 2019). Like many coastal environments our study site in Placentia Bay Newfoundland, located in the Northwest Atlantic, is exposed to multiple stressors including rising sea surface temperatures, storm events, tanker traffic, and aquaculture (Kiceniuk 1992; LGL 2018; Chen et al. 2020). Placentia Bay is an Ecologically and Biologically Significant Area (EBSA) which features eelgrass habitat, capelin spawning beaches, salmon rivers, marine mammal and leatherback turtle habitats, seabird colonies, as well as important coral and sponge areas (Wells et al. 2019). In the marine environment, soft-sediment benthic macrofauna (herein defined as organisms retained on a 500 μm screen) can exhibit high diversity in seafloor sediments and contribute to a variety of essential ecosystem functions including acceleration of organic matter decomposition, bioturbation, and filtration (Covich et al. 1999; Chen 2021). Macrofauna are an important food source for many species and play a vital role in nutrient cycling and energy flow (Covich et al. 2004; Belley and Snelgrove 2016; Snelgrove et al. 2018; Drejou et al. 2021). Given these attributes benthic macrofauna are common biological indicators used in ecological assessments of coastal environments worldwide (Borja et al. 2000; Van Hoey et al. 2010; Ramey 2011).
The distribution and community structure of macrofauna are influenced by several abiotic and biotic processes. Given the intimate association that soft-sediment macrofauna have with their sedimentary habitat, differences in sediment grain size, organic content, stability, porosity (e.g., Rhoads and Young 1970; Snelgrove and Butman 1994; Snelgrove et al. 2018), and the presence of hydrogen sulfide (Vaquer-Sunyer and Duarte 2010), pH (Clements and Hunt 2018a, b; McGarringle and Hunt 2024), and contaminants (Reynoldson 1984) contribute to the well-known, characteristically patchy distributions of benthic communities. Hydrodynamic conditions or water velocity near the seafloor can influence sediment type which in turn influences sediment geochemistry (Clements and Hunt 2018a, b). Fine sediments (silt; 3.9–63 μm grain size) generally occur in areas of weak flow and allow for greater deposition of organic material, whereas coarser sediments (sand; 63–2000 μm grain size) are present in fast-flowing waters with frequent sediment resuspension (Dyer 1986; Ramey and Bodnar 2008). Fine sediments consequently tend to be richer in organic content resulting from both decreased water velocity, as well as their enhanced ability to bind organic carbon (Snelgrove and Butman 1994). In the Bay of Fundy Canada (Clements and Hunt (2018b) found that sites with coarser sediments also had higher porewater pH than sites with muddier sediment, likely due to increased water penetration into coarser sediments having larger interstitial spaces. The feeding mode of macrofauna is also influenced by hydrodynamics. Generally, deposit feeders have greater abundance in silty/muddy sediments, whereas suspension feeders are associated with coarse sands (Snelgrove and Butman 1994).
Large scale, oceanographic conditions (km to 100s m) such as temperature, salinity, surface productivity, and circulation are also important factors influencing coastal benthic communities (e.g., Barry and Dayton 1991; Morrisey et al. 1992; Olafasson et al. 1994; Lima and Wether 2012; Alexander et al. 2018). A recent study examining warming based on sea surface temperature (SST) anomalies, in the North Atlantic (i.e., at St. John’s, Newfoundland [NL] Canada) projected an increase of 0.4–2.2 °C over the next 50 years (Han et al. 2015). Spatio-temporal SST gradients are important in influencing wind patterns, storm development, and wave activity through ocean-atmosphere interactions (Bengtsson et al. 2006; Reguero et al. 2019). Attributed to rising SST (C2ES 2020), the North Atlantic Basin has seen an increase in the intensity of tropical storms including hurricanes (Méndez-Tejeda and Hernández-Ayla 2023). Moreover, wave activity in terms of wave height has also increased at high latitudes in recent decades (Reguero et al. 2019). Physical disturbance to the benthic habitat by storms and wave action have the potential to influence seafloor substrates (Herkül et al. 2016) through erosion and deposition of sediments. Disturbance from storms can also affect circulation patterns (Ma et al. 2017), salinity, and concentrations of dissolved oxygen (Montagna 2023). Lower salinity and dissolved oxygen caused by storm events can affect the abundance and diversity of benthic macrofauna with recovery times ranging from 3 months to 3 years (Mallin et al. 2002; Montagna 2023). For example, in San Antonio Bay Texas, Montagna (2023) observed a 71% decline in macrofaunal abundance and a 54% decline in species/taxon richness after Hurricane Harvey impacted Texas as a category 4 storm. Moreover, increasing water temperature can influence species distributional ranges leading to alterations in community structure. For example, species accustomed to warmer waters may be able to expand into warming temperate regions, and cooler water species may retreat northward. A study conducted along the Atlantic coast in the USA, looking at the Carolinian (Cape Canaveral, Florida and Cape Hatteras, North Carolina) and the northern Virginian boundary (Cape Cod) provinces, recorded 22 species of benthic macroinvertebrates limited to southern waters had shifted their range northward likely due to water warming (Hale et al. 2017).
Changes in species distribution and environment, especially in terms of sediment type and food resources, can in turn affect the essential ecosystem functions supplied by macrofaunal communities through differences in the biological traits of the different taxa present in the community (Rand et al. 2018; Sutton et al. 2020). Overall, biodiversity (e.g., measured as species richness) has been recognized as an important factor in maintaining ecosystem functioning and the many services associated with the coastal environment (Balvanera et al. 2006; Muntadas et al. 2016). Biodiversity loss can reduce a community’s ability to recycle biologically essential nutrients emphasizing the need to understand the link between biodiversity and ecosystem functioning (Cardinale et al. 2012; Belley and Snelgrove 2016). A functional trait approach, which characterizes communities based on the functional roles of species rather than taxonomic identity, can allow for a better understanding of how communities use resources as well as their resilience to environmental change (Sutton et al. 2020). For instance, trait redundancy (e.g., species performing similar functional roles) is thought to increase temporal stability by dampening the effect of species loss via function retention (Walker 1992; Biggs et al. 2020). Previous studies have shown that functional richness (i.e., the amount of ecological niche space occupied by species in a community) is a better predictor of benthic flux (i.e., changes in oxygen and nutrients) than species richness (Belley and Snelgrove 2016).
Using historical and contemporary benthic macrofaunal composition and abundance data (1998 vs. 2019/2020 respectively) taken 21 years apart, the main objective of the present study was to investigate changes in community and functional structure in Placentia Bay, NL, and examine environmental drivers of the observed variation. It was hypothesized that: (1) historical and contemporary communities in the bay would be different and community patterns would be related to the sedimentary habitat, as it can be influenced by larger-scale oceanographic features such as SST (e.g., Herkül et al. 2016). Sea surface temperature has been increasing at a high rate in the North Atlantic (Alexander et al. 2018) and can also change macrofaunal species distribution and community composition (e.g., Hale et al. 2017), (2) functional structure (macrofaunal trait expression) would be related to community structure (taxonomic composition and abundance) such that they would exhibit similar spatio-temporal patterns of variability (e.g., Sutton et al. 2020), and (3) there would not be a loss of functional traits/modalities between historical and contemporary communities such that functional roles should remain fulfilled (i.e., no loss of modalities) allowing for any loss of species/taxa to be buffered against (Walker 1992; Frid and Caswell 2015; McLean et al. 2019).
Methods
Study Area
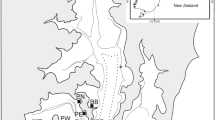
Placentia Bay is the largest embayment on the southeast coast of Newfoundland (47.1030 °N, 54.1859 °W) (Fig. 1). The bay is ~ 130 km long and 100 km wide at its southerly directed mouth that is open to the Atlantic Ocean. The average depth in the bay is 125 m (Ma et al. 2012). The inner part of the bay contains three channels divided longitudinally by several islands (Ramey and Snelgrove 2003). Bottom salinity in the bay ranges from 32.2 to 33.0 (Ramey 2001; Ramey and Snelgrove 2003). Currents are generally influenced by local factors such as wind, water density, and seafloor topography (Bradbury et al. 2000; Ma et al. 2012). In the spring and fall, prevailing winds are from southwest and west, whereas in the winter winds are from northwest to west (Ma et al. 2012) which can have an effect on surface productivity (Van Ruth et al. 2010). Prior to the present study, macrofaunal communities in Placentia Bay were only examined in 1998 when Ramey and Snelgrove (2003) conducted the first comprehensive sampling at ten sites distributed throughout different regions of the bay and on the shelf (hereafter referred to as “historical”: Ramey 2001; Ramey and Snelgrove 2003). Eight of these sites were sampled in 2019 and 2020 as part of an ecological baseline study for Placentia Bay (hereafter referred to as “contemporary”) (Fig. 1). Offshore sites sampled in 1998 (shelf sites 2 and 3) were not resampled in 2019/2020 because sampling for the contemporary research was focused in the inner bay (leaving 8 sites of the original 10), and the research vessel did not go beyond the point of the shelf 1 (S1) site.
Placentia Bay, Newfoundland showing sampling sites H1 (head of bay), E1/E2 (eastern channel), W1/W2 (western channel), C1 (central channel), O1 (outer bay), and S1 (continental shelf), also see Table 1. Inset shows Placentia Bay in relation to Newfoundland
Sample Collection
Contemporary sample collection was conducted in 2019 and 2020 (i.e., 14–16 September 2019 and 13–17 October 2020) at eight sites in the bay using a 0.1 m2 Van Veen grab (Department of Fisheries and Oceans, Canada, NL) (Fig. 1). Three to six independent grab samples were haphazardly collected at each site (total n = 36), (Table 1). Grab samples were subsampled (50 ml of sediment removed) for determination of sedimentary variables including grain size and total organic matter (top 5 and 1 cm of sediment respectively) (n = 36). Additional samples for sediment grain size were also collected in 2021 (September 21–October 2).
Historical samples used for comparison were collected in 1998 (June and July), from the same eight sites using a random, nested design (Ramey 2001; Ramey and Snelgrove 2003) (Fig. 1; Table 1). Samples were obtained with a rectangular single-spade box corer containing six subcores. A total of six box cores were collected at each site in the bay, with fewer samples in the outer bay and shelf (total n = 41; Table 1). For each box core, the four subcores (10 cm × 10 cm wide to a depth of 10 cm; 4 × 100 cm2 = 400 cm2) were used to examine the macrofaunal community and one was used for sediment analyses (Ramey and Snelgrove 2003). Sediment cores were subsampled for total organic carbon (TOC) (i.e., all cores, n = 38) and grain size (i.e., three random cores per site, n = 22) (Ramey and Snelgrove 2003). The four macrofaunal subcores were pooled for community composition analysis since subcores were not considered independent given that they were not completely separated from each other (Ramey and Snelgrove 2003). Any Van Veen and box core samples that were washed out or where the top sediment layer appeared to be disturbed were not retained and were re-sampled.
Sample Processing
All macrofaunal samples were fixed in 4% buffered formalin and then transferred to 70% denatured ethanol and sieved over a 500 μm mesh screen before sorting macrofauna from the sediments using a stereomicroscope (all years). Macrofauna were counted and identified to the lowest practical taxonomic level, usually species.
For grain size analysis, sediments were treated with water/peroxide solution to remove organic material. Sediments were then dried and weighed followed by resuspended and disaggregated before sieving to separate the sediment grains into coarse (sand: >63 μm), fine (silt: <63–3.9 μm), and clay (< 3.9 μm) sized fractions (Wentworth scale), (see Ramey and Snelgrove 2003: historical; Danovaro 2009 and adapted by Bureau Veritas: contemporary). The percent sand, silt, and clay in each sample was calculated using dry weights.
For determination of total organic matter (TOM) of contemporary samples, 50–100 mg of dried sediment (historical freeze dried − 60 °C; contemporary 105 °C) was weighed and placed in a muffle furnace for 4 h at 450 °C. The TOM was the difference between dry and calcinated sediments which was normalized and expressed as a percentage (Danovaro 2009). For the historical samples, total organic carbon (TOC) values from Snelgrove and Ramey 2003 were multiplied by 1.724 to determine TOM, as this is a commonly used conversion factor based on the assumption that most soils consist of ~ 58% organic carbon (Nelson and Sommers 1996; Pribyl 2010).
Data Analysis
Macrofaunal Data
Only infaunal, soft-sediment macrofauna (organisms > 500 μm) were included in community analyses following Gallagher and Grassle (1997); Taghon et al. (2017a). Hard substrate epifaunal encrusting species (e.g., bryozoans, mussels, and hydrozoans) and highly mobile species (e.g., mysids and decapods) not reliably sampled using a Van Veen grab or box corer were also excluded (Taghon et al. 2017a). To take a conservative approach in comparing the historical and contemporary community structure, rare species (species/taxa with < 10 individuals across all years and samples) were also removed (Taghon et al. 2017a). The taxonomic status of species was checked against the continuously updated list in the World Register of Marine Species (http://www.marinespecies.org/index.php). To ensure consistent species/taxon identifications between the contemporary and historical datasets, reference specimens (i.e., 48 commonly abundant taxa: P. Ramey-Balci pers. collection), and identification notes and images contained in Ramey et al. (2001) were compared with contemporary reference specimens. This ensured that species/taxon differences between the two data sets were not a result of discrepancies in identification. On occasion, when identifications could not be confirmed and the level of taxonomic identification varied between the two datasets, taxon/species identifications were grouped to a common higher level of classification. The sample size/amount of sediment collected by the different gear types varied for the historical versus the contemporary samples (i.e., historical: box corer, 0.04 m2; contemporary: Van Veen grab, 0.1 m2) and therefore species densities for the historical data were re-scaled from 0.04 m2 to 0.1 m2 for data analyses.
Functional Traits
A total of eight traits subdivided into 36 modalities were used to describe the functional roles of macrofauna in the bay (Table S1). The functional traits selected describe morphological (i.e., body size), behavioral (i.e., adult movement, bioturbation, living habitat), life history (i.e., reproduction, larval development), and physiological (i.e., feeding mode, tolerance) attributes of macrofauna that directly or indirectly play a role in important ecosystem functions such as nutrient cycling and sediment transport (Martini et al. 2020). Species/taxa for a given modality were scored using the “Fuzzy coding” procedure (Chevenet et al. 1994; Bremner et al. 2006; Oug et al. 2012) based on the following scale, where 0 = no affinity (modality not employed), 1 = low affinity, 2 = high affinity, and 3 = exclusive affinity (Oug et al. 2012). For example, when looking at a trait such as feeding mode, species can have more than one modality (e.g., deposit and surface feeding), in this case, “1” would be assigned to the less commonly employed modality and “2” to the more commonly employed modality (Oug et al. 2012). If the species/taxon only used one feeding mode (e.g., deposit feeding) a value of 3 was assigned. Modality scores for each trait were assigned based on available databases including the Arctic Trait Base (https://arctictraits.univie.ac.at/), which contained the large majority of the species/taxa in Placentia Bay consistent with its geographic location, as well as The World Register of Marine Species (WoRMS, https://www.marinespecies.org/), and Polytrait database (http://polytraits.lifewatchgreece.eu/) which were accessed in 2022. This produced a species by trait/modality matrix whereby the modality scores for the species/taxa were multiplied by their abundance in each sample (no. ind. 0.1 m−2 ), and then summed over all species/taxa to obtain a single value for each modality in each sample producing an abundance weighted, functional trait expression matrix for analysis (Bremner et al. 2006; Oug et al. 2012).
Multivariate Statistics
Variation in macrofaunal community structure and environmental variables between the historical (1998) and contemporary years (this group includes individual samples taken in 2019 and 2020; see Table 1) were examined by conducting Principal Coordinates Ordination (PCO) analysis in PRIMER (v7 +PERMANOVA) (Anderson 2001, 2017; Clarke and Gorley 2015). Samples collected in 2019 and 2020 were grouped as “contemporary” since community metrics and multivariate analysis of species/taxon data for 2019 vs. 2020 were not different (see Komendić 2023). For the macrofaunal data, the PCO was conducted on a Bray-Curtis dissimilarity matrix generated from species/taxon composition and abundance (at the level of sample; n total = 77). Prior to analysis, abundances were fourth root transformed to downweigh the relative influence of the more abundant species/taxa, which would otherwise tend to dominate the dissimilarity matrix (Clarke and Gorley 2015). Bray-Curtis dissimilarity shows the proportion of differences in abundance between the samples where 0 indicates samples are similar and 1 indicates samples are completely dissimilar (Legendre and Legendre 2012). Species/taxa with a correlation coefficient of ≥ 0.7 (Pearson correlation) with the ordination pattern were overlaid as vectors on the PCO plot, where arrows point in the direction of maximal variation in species abundances, and their length is proportional to their maximal rate of change (Ramette 2007). A Permutational Multivariate Analysis of Variance (PERMANOVA; 9999 permutations) tested the null hypothesis that there is no difference in the centroids (equivalent to mean in univariate analysis) in communities between time points (historical vs. contemporary years; n = 77). Site (and site x time interaction) was also included in the model using the Type III Sum of Squares (SS) for unbalanced designs so that each factor was fit only after taking into account all other terms. This ensured complete independence of each term in the model (Anderson et al. 2008) as we were focused on examining the main effect of time (historical vs. contemporary) independent of site differences in the present study. Ramey and Snelgrove (2003) and Komendić (2023) examined community structure in relation to spatial scale differences among sites in the bay). Similarity percentage (SIMPER, Clarke 1993) was used to identify the species/taxa contributing to 50% of the dissimilarity between years. These analyses were also conducted to examine the composition of species/taxa (i.e., presence/absence) between years whereby PCO was conducted on a Sorensen resemblance matrix.
Principal Coordinates Ordination analysis (which is equivalent to PCA when based on Euclidean distance) was also used to examine differences among samples based on the sedimentary habitat (i.e., grain size, TOM). Draftsman plots (i.e., plots of each variable against each other variable) were not strongly skewed and therefore environmental variables did not need to be transformed prior to analysis (Clarke and Gorley 2015), however, all variables were normalized to Z-scores to weigh variables equally (i.e., places them on the same scale). This reduces the degree to which any one variable with a larger mean value sways the result. A PCO analysis was run on this matrix based on Euclidean Distance. A PERMANOVA (9999 permutations) tested the null hypothesis that there is no difference in the sedimentary habitat between the historical vs. contemporary years as outlined above for species/taxon abundance.
To examine the relationship (correlation) between the patterns observed for the sedimentary environmental data compared to the macrofaunal data the “RELATE” function in PRIMER v7 was used (Clarke et al. 2014; Clarke and Gorley 2015). This analysis tested the null hypothesis that there is no correlation (Spearman rank matching coefficient rho = 0, with 999 permutations) between the environmental data matrix (i.e., distance-based) vs. the biological data matrix (i.e., for both the Bray-Curtis dissimilarity generated from species/taxon abundances and the Sorensen metric-based taxon presence/absence). To determine how much of the observed variation in the macrofaunal data could be explained by the sedimentary environment a distance-based linear model (DistLM) was performed using percent sand, silt, and TOM as predictor variables and macrofaunal data/resemblance measures as the response. This analysis is a permutation-based method that does not assume normally distributed data and is a form of multivariate multiple regression conducted on distance and/or dissimilarity matrices (Anderson et al. 2008). Predictor variables were added to the model following the stepwise procedure and the AICc selection criterion was used to evaluate the models. Prior to this analysis, examination of correlations between predictor variables indicated that silt was correlated with both sand and TOM (Pearson r=-0.609, n = 57 p < 0.001 and Spearman rho=-0.576, n = 57, p < 0.001, respectively). Both variables were retained in the model since they were below the threshold of r ≥ 0.95 whereby they would be considered to effectively contain the same information and thus redundant for purposes of analysis (Legendre and Anderson 1999).
To compare functional traits between the historical and contemporary years and identify environmental drivers of observed patterns, the same multivariate analyses conducted for the macrofaunal data were repeated for the functional traits with the exception that the PCO was conducted on the Canberra dissimilarity metric generated from the abundance weighted, functional traits expression matrix (instead of Bray-Curtis dissimilarity) to overcome arch effects seen in exploratory analysis via PCO and metric multidimensional scaling (MDS) (Podani and Miklós 2002). The “Relate” function in PRIMER v7 examined the relationship between the macrofaunal vs. functional trait data, as well as the environmental variables vs. the functional trait data (for both the Canberra metric generated from expression of functional traits and Sorensen metric based on presence/absence of modalities) (Clarke et al. 2014; Clarke and Gorley 2015). These analyses tested the null hypotheses that there is no correlation between the observed patterns in the macrofaunal community data vs. functional trait data, and between the environmental vs. functional traits data). To determine how much of the observed variation in the functional trait data can be explained by the sedimentary environment a DistLM was performed using percent sand, silt, and TOM as predictor variables and functional trait data/resemblance measures as the response.
Community Metrics and Univariate Statistics
To assess if the sampling effort was adequate, species accumulation curves were generated separately for the historical and contemporary samples considering all species/taxa, as well as the major taxonomic groups (i.e., Amphipoda, Bivalvia, Gastropoda, Polychaeta) (PRIMER v7). Pie charts examined the proportion of major taxonomic groups (i.e., percent total abundance) making up the communities in the historical versus contemporary. To compare species composition between time points, the percentage of species/taxa (out of the total species richness for the bay) only sampled in the historical, contemporary, or shared between them were plotted using stacked bar plots. Boxplots compared community metrics including macrofaunal density (no. of ind. 0.1 m−2), richness (e.g., number of species/taxa), evenness (Pielou’s Evenness Index, J’), and diversity (Shannon-Wiener, H’ log e) as well as sedimentary variables (i.e., sand, silt, TOC) between years. Student t-tests examined differences in community metrics, abundance of major taxonomic groups, and sediment variables between years. Given the importance that TOM can have in influencing macrofaunal abundance, a linear regression tested if TOM predicted the abundance of polychaetes (given they were the dominant taxon) as well as other major groups (i.e., pooled abundances for amphipods, gastropods, and bivalves) for each time point. When the data were normal and had equal variance or could be normalized through transformation (i.e., log or square root) a t-test was directly employed. On occasion when transformations could not normalize the data, a t-test was used when the non-parametric Wilcoxon (Mann-Whitney U) test produced the same result; given that t-tests are robust against non-normality (Rasch et al. 2007). The normality of the distribution of the above datasets was tested using Shapiro-Wilk test and equal variance was examined with Levene’s test of homogeneity. All univariate statistical analyses were performed in JASP (V. 0.16, JASP Team 2022). For functional traits, the proportion of each functional modality (i.e., percent of total expressed traits), as well as the proportions of modalities making up each of the eight traits (such that the values of all modalities for a particular trait add to 100%), were compared for the historical and contemporary samples with the later displayed as circular bar plots (“corrplot” package in R Studio; Wei and Simko 2017).
Results
Community Structure
The dataset contained 36,909 individuals and 77 species/taxa for analysis. The historical samples included 30,663 individuals (based on standardized counts to 0.1m2 ) and 64 species/taxa, whereas the contemporary data contained 6,246 individuals (61 species/taxa). Although more samples were collected in the historical (n = 41) compared to the contemporary years (n = 36) the total area of the seafloor sampled was greater for the contemporary (i.e., 1.64 m2 vs. 3.6 m2 respectively) (Table 1). Overall, the major taxonomic groups including the Amphipoda, Bivalvia, Gastropoda, and Polychaeta made up 90% of the total abundance and > 80% of the species/taxa sampled in the bay (historical and contemporary data combined). Species/taxa accumulation curves for the historical and contemporary samples leveled off at ~ 36 and ~ 29 samples respectively (Fig. 2) and curves for the four major taxonomic groups (except amphipods) also reached an asymptote (Fig. S1a–d). Principal Coordinates Ordination (PCO) analysis of species/taxon composition and abundance explained 44.2% of the total variability in the data with the historical and contemporary samples generally forming two groups (Fig. 3a). A total of 18 species/taxa including 12 polychaetes and 3 bivalves, contributed ~ 50% of the dissimilarity between these time points, with the highest contribution from the polychaete C. pygodactylata (6.74%) (Table 2). Note that C. pygodactylata was previously identified as Cossura longocirrata (see Ramey and Snelgrove 2003). Variation in macrofaunal community structure between the historical vs. contemporary samples differed (independent of site) (PERMANOVA, pseudo-F1, 61=27.1, p < 0.001) (Fig. 3a, Table S2) and by site (independent of time) (pseudo-F7, 61=9.2, p < 0.001) with an interaction (Table S2). Results were consistent even when the dominant species, C. pygodactylata was removed from this analysis (Table S2). Similarly, PCO analysis of species/taxon composition (i.e., presence/absence; 45.6% of the total variability explained) differed between the historical vs. contemporary samples independent of site (PERMANOVA, pseudo-F1, 61=23.7, p = < 0.001) (Fig. 3b) and by site (independent of time) (pseudo-F7, 61=9.2, p < 0.001) (Table S2).
Comparison of the major taxonomic groups between the historical and contemporary samples showed the polychaetes made up the greatest proportion of macrofauna collected in both years, however, their proportion was much higher in the historical samples (91 vs. 58% respectively) (Fig. 4, also Table S3). In contrast, the proportion of amphipods, bivalves, and gastropods were relatively higher in the contemporary samples (Fig. 4; also Table S3). Moreover, examination of the species making up these major groups found 41 species/taxa (63%) shared between the historical and contemporary years, whereas 14 species/taxa (22%) were only present in the historical samples and 10 species/taxa (15%) were unique to the contemporary (Fig. 5, also Table S4). Mean density of individuals making up the major groups were not different (Gastropods: t75 = 0.1, p = 0.325, Bivalves: t75 = 1.7, p = 0.093, Amphipods: t75 = 0.5, p = 0.616) for the historical vs. contemporary, except for polychaetes which were 6.5 times higher (t75=-6.7, p < 0.001) in the historical (680 ± 80 ind. 0.1m−2, n = 41 vs. 100 ± 17 ind. 0.1m−2, n = 36 respectively) (Fig. 6). All community metrics examined, (except for species/taxon richness) differed between years (Fig. 7). Species/taxon evenness (J’) and Shannon diversity (H’) were both lower (t75 = 7.1, p = < 0.001 and t75 = 6.4, p = < 0.001 respectively) in the historical relative to the contemporary samples (evenness: 0.56 ± 0.03, n = 41 vs. 0.80 ± 0.01, n = 36; diversity: 1.70 ± 0.09, n = 41 vs. 2.40 ± 0.05, n = 36; respectively) (Fig. 7). Macrofaunal density was higher (t75=-7.7, p < 0.001) in the historical (748 ± 85 no. ind. 0.1 m−2, n = 41) vs. the contemporary samples (174 ± 20 no. ind. 0.1 m−2, n = 36) (Fig. 7).
Proportion (%) of total number of species/taxa in samples that were unique to historical (black fill) or contemporary (white) samples or shared (gray) between them for each major taxonomic group (i.e., Amphipoda, Bivalvia, Gastropoda, and Polychaeta). Numbers on bars indicate number of species/taxa shared or unique to each time point
Boxplots comparing a density (total number of individuals 0.1 m−2), b evenness (J’), c species richness (number of species/taxa 0.1 m−2), and d Shannon diversity (H´log e) for historical (n = 41) versus contemporary (n = 36) communities. Line dividing the box in half represents median and X represents mean. Vertical spread of the box depicts interquartile range encompassing the middle 50% of data. Dots denote outliers. *= means differed, full statistical results provided in main text
Environmental Data
Principal Coordinates Ordination (PCO) analysis of the environmental variables explained 91.9% of the total variability in the data. The sedimentary environment differed (PERMANOVA, pseudo-F1, 41=90.6, p < 0.001) between these two-time points and between sites (pseudo-F7, 41=9.8, p < 0.001) with an interaction (Fig. 8, Table S2). Examination of sediment grain size showed that the proportion of silt was higher (t55 = 8.6, p < 0.001) in the historical (58 ± 2.3%, n = 22) compared to the contemporary samples (37 ± 1.3%, n = 35), whereas the proportion of sand in the historical samples was lower (t55 = 9.0, p < 0.001) (6 ± 1.40%, n = 22 vs. 27 ± 1.50%, n = 35) and sediments have become consistently coarser at nearly all sites sampled in the bay, from 2019 to 2021 (Fig. 9a, b). Total organic matter was also lower (t72=-2.8, p = 0.006) in the historical (10.4 ± 0.76%, n = 38) compared to contemporary samples (13.5 ± 0.98%, n = 36) (Fig. 9c).
Historical versus contemporary sediment data including a box plots comparing percent sand, silt, and clay (n = 57), b bar plot showing mean ± SE percent sand among sites for four-time points 1998–2021 (n = 60), and c percent total organic matter (n = 57). Line dividing the box in half represents median and X represents mean. Vertical spread of the box depicts interquartile range encompassing the middle 50% of data. Dots denote outliers. Sites without bars = no data. For a, c *=means differed, full statistical results provided in main text
Functional Traits
Principal Coordinates Ordination (PCO) analysis for the expression of functional traits explained 75.2% of the total variability in the data (Fig. S2a). The expressed functional traits differed (PERMANOVA, pseudo-F1, 61=21.2, p < 0.001) between the historical and contemporary samples and between sites (pseudo-F7, 61=4.2, p < 0.001) (Table S2). The spatio-temporal patterns were similar to those observed for species/taxon abundances (RELATE, rho = 0.55, p = 0.001). Ten modalities related to tolerance, body size, feeding, bioturbation, reproduction/larval development, adult movement, and living habitat contributed to ~ 50% of dissimilarity between the two-time points (Table S5). Examination of modalities making up at least 3% of the total expressed traits for either of the two time points, showed that historical samples contained a relatively higher proportion of highly tolerant (8% vs. 4%, historical vs. contemporary respectively), small body sized (< 10 mm, 9% vs. 4%), subsurface deposit feeders (9 vs. 4%), surface deposit bioturbators (8% vs. 5%), and direct developers (9 vs. 5%) (Fig. 10). These modalities, however, were still proportionately most abundant in the contemporary samples, except for small-medium body size and surface deposit feeders (Fig. 10). Overall, examination of the relative proportion of different modalities making up each trait, showed that the reduced expression of dominant modalities in the contemporary samples (i.e., modalities comprising > 50% of the total expressed modalities for their respective trait in the historical), allowed for a more even distribution of modalities among the respective traits compared to the historical time point (Fig. 10b). In contrast, the composition of functional traits (i.e., presence/absence of modalities) between the two years and sites was similar (Fig. S2b, and Table S2).
Comparison of functional traits between the historical versus contemporary samples a stacked bar plots for modalities making up at least 3% of the total expressed traits, and b proportion (%) of modalities making up each of the eight traits. Trait modality definitions defined in Table S1. Note: for b modalities for each trait sum to 100%
Environmental Drivers
Patterns observed in the macrofaunal resemblance matrix including Bray-Curtis dissimilarity generated from species/taxon abundances and Sorensen based on taxon presence/absence matched those found in the environmental resemblance matrix (RELATE, rho = 0.38, p = 0.001 and rho = 0.36, p = 0.001 respectively). The DistLM indicated that the combination of silt, sand, and TOM predicted patterns in species/taxon abundance (25% of variance explained) and composition (i.e., presence/absence; 23% of variance explained) (Table 3). Overall, silt explained the largest proportion of the variance (15 and 13% respectively). Patterns observed in the functional trait resemblance matrix (Canberra metric generated from the expression of functional traits) were also correlated to those found in the environment resemblance matrix (RELATE, rho = 0.25, p = 0.001). Silt and sand predicted observed patterns with silt explaining the largest proportion of the variance 23% (total explained 27%) (Table 3). In contrast, patterns based on the functional trait resemblance matrix (Sorensen; generated from presence/absence of modalities) did not match those observed in the environmental resemblance matrix (RELATE, rho = 0.01, p = 0.54) and the environmental variables were not predictors of the presence/absence of functional traits. For both historical and contemporary samples, a linear regression indicated that TOM predicted polychaete abundance (r2 = 0.26, F1,36=12.9, p < 0.001 and r2 = 0.14, F1,33=5.4, p = 0.027 respectively) (Fig. S3a) as well as the abundance of other major groups (i.e., pooled abundances for amphipods, gastropods, and bivalves) (r2 = 0.15, F1,36=6.3, p = 0.017 and r2 = 0.34, F1,33=17.1, p < 0.001, respectively) (Fig. S3b).
Discussion
The macrofaunal community and functional structure, and sedimentary habitats in Placentia Bay have undergone significant changes since 1998. Overall, 14 species/taxa were only observed in the historical community, whereas 10 species/taxa were unique to the contemporary. While 63% of species/taxa making up the major taxonomic groups were shared between these two-time points, this contrasts with 86% shared between the two contemporary years (2019 vs. 2020) which were also shown by Komendić (2023) to be similar in their community structure. Herein, the contemporary community exhibited a lower density of macrofauna but had similar richness, resulting in higher evenness and diversity compared to the historical one. Community and functional structure in the bay were related to corresponding changes in the sedimentary habitat, known to be important in structuring benthic communities. Most notably there have been changes in sediment grain size and total organic matter content such that in contemporary years grain size is coarser and organic matter is higher relative to 1998. While spatio-temporal patterns of variability based on functional expression of macrofaunal traits reflected those in taxonomic composition and abundance, functional composition (i.e., presence/absence of modalities/traits) did not vary between the historical and contemporary communities, despite changes in taxonomic composition. Similarly, Sutton et al. (2020) found that epibenthic communities in two Alaskan Arctic shelfs were more similar in terms of their functional and taxonomic diversity than in functional and taxonomic composition. Such findings (including those of the present study) lend some support to the idea that redundant functional traits (implying ecological functioning) may be able to be maintained under changing species composition as taxa being eliminated are replaced by others with similar traits (Frid and Caswell 2015; McLean et al. 2019). However, changes in the relative expression of functional traits are also important to consider as they can influence which resources are used and how effectively different services can be maintained (Cadotte et al. 2011; Sutton et al. 2020).
Although temporal samples taken at specific time points such as decades apart have inherent limitations that make community variability difficult to interpret (Renaud et al. 2007; Herder et al. 2021) such comparisons are important in understanding and predicting current and future community changes (e.g., Van Geest et al. 2015; Taghon et al. 2017a; Harris et al. 2023; Ramón et al. 2023). Therefore, it is pertinent to consider differences in sampling methods and protocols when making comparisons and interpreting results (Taghon et al. 2017a). In our case, yearly sampling effort and sieve size were consistent over years. Furthermore, taxonomic discrepancies were clarified between the two datasets using existing reference collections. While the grab used to collect the contemporary samples was larger than the box core used for the historical sampling, species/taxon richness was similar and the density of macrofauna was lower in the contemporary samples (even before the scaling of historical samples to facilitate comparisons); which is the opposite trend than one might expect based on sample size alone. Species densities determined using a Van Veen grab vs. box core samples in fine sands have been shown to be comparable, except for deeper living species when the Van Veen does not penetrate past the top 5 cm of sediment (Beukema 1976). In our study, the average penetration depth of the Van Veen grab was 12.6 ± 4.1 cm.
Species accumulation curves generated separately for both time points showed that the overall community as well as the major taxonomic groups were adequately sampled (apart from amphipods). Thus, an increase in species richness would not be expected with additional sampling. We did however have a discrepancy with respect to sampling season which can result in marked changes in species abundances in temperate regions (Taghon et al. 2017a) especially in the summer (following the reproductive period) compared to winter (Reiss and Kröncke 2005). For example, at two shallow sites (10 m and 30 m depth) in the temperate/subarctic Baltic Sea, the abundance of macrofauna (500 μm sieve) peaked in late July/August and was lowest in the winter, while density in June was more similar to the winter (Kauppi et al. 2017). In contrast, other studies have not found changes in macrofaunal abundances with season (e.g., North Atlantic: Valderhaug and Gray 1984; Weissberger et al. 2008; Arctic: Mazurkiewicz et al. 2019). While season in the present study may have contributed to some of the variability between years as the contemporary samples were collected in September/October vs. June/July for the historical, sediment grain size and TOM predicted patterns in macrofaunal species/taxon composition and abundance. Moreover, lower abundance in the fall of 2019/2020 was taxon-specific being only observed for the polychaetes and considerable changes in community composition (i.e., presence/absence of species/taxa) were also observed (even after the removal of rare species); which would be less likely to vary substantially with season (e.g., Mazurkiewicz et al. 2019).
More specifically, in terms of changes in the abundance of polychaetes, Cossura pygodactylata, Dorvilleidae spp., and Gyptis bruneli in particular, showed large reductions (> 50%) in their mean densities in the contemporary community relative to the historical one. Cossura pygodactylata (Family Cossuridae) was the most abundant species present at both time points and this family has been observed to vary seasonally. Weissberger et al. (2008) examining macrofaunal communities in the Gulf of Maine found the density of cossurid polychaetes was lowest in the fall but despite this, it remained a dominant taxon. Similarly in the present study, even with the lower density of C. pygodactylata in the contemporary community (sampled in the fall), it remained the most dominant species in the bay, and community structure between the historical vs. contemporary time points remained statistically different even when it was removed from the multivariate analyses. In contrast, bivalves, amphipods, and gastropods showed the opposite trend, whereby their relative proportions and abundances tended to be higher in contemporary relative to the historical community. Bivalves were also responsible for some of the observed dissimilarity between the two-time points.
The sedimentary habitat in Placentia Bay has also undergone significant changes in sediment type. This contrasts with studies examining changes in sediment type over time that have indicated that grain size tends to remain relatively stable (Taghon et al. 2017a; Herder et al. 2021). For example, Taghon et al. (2017a) found that sediment properties in Barnegat Bay, New Jersey, showed few changes in 45 years such that the silt/clay content was similar in 1965-69, 2000-06, and 2012-14. Likewise, a previous long-term study in Frobisher Bay, Nunavut, Canada, found that grain size did not change over 50 years (i.e., between 1967 and 1976 and 2016) (Herder et al. 2021). In Placentia Bay, contemporary sediments were ~ 4.5x coarser as compared to the historical time point. As physical disturbance created by storms can modify seafloor substrates (Herkül et al. 2016) it is plausible that changes in sediment grain size in the bay may be related to storm activity. Newfoundland is subject to numerous extratropical cyclones (Xu et al. 2019) which are a main cause of storm surge events (Xu et al. 2019; Orton et al. 2016). Igor in 2010, was the most intense extratropical cyclone affecting NL to date (Ma et al. 2017). For the contemporary time points two storms occurred before infaunal sampling in 2019/2020 (i.e., ~ 5–9 days after extratropical cyclone Dorian in 2019 and ~ 21–25 days after post-tropical storm Teddy in 2020), and the collection of samples for grain size in 2021 (i.e., ~ 1–12 days after extratropical cyclone Odette). These storms variably affected Atlantic Canada including NL through storm surges, strong winds, waves, and heavy precipitation (Avila et al. 2020; CBC News 2020; Cangialosi 2021). Disturbance by waves and currents during storm events can cause sediment transport (i.e., deposition), as well as removal (i.e., erosion) in coastal regions (Manchia et al. 2023). While Taghon et al. (2017b) found no change in sediment median particle size after Hurricane Sandy, it is possible that sediments in Barnegat Bay were not affected because the bay is protected by two barrier islands that shelter it from the Atlantic Ocean waters. In contrast, Placentia Bay has a large mouth (100 km wide) and no prominent sill, exposing it to the adjacent shelf environment allowing significant exchange of water with the Atlantic Ocean (MacIsaac et al. 2023). Placement of current meters at some of the established study sites would aid in determining whether bottom flows during storm events are a plausible mechanism for the observed changes in sediment type. Additional sampling in the bay is needed to determine whether the bay has undergone sustained changes in sediment grain size, however, sediments have become consistently coarser at nearly all sites sampled in the bay from 2019 to 2021. Indeed, the comparison of habitats and communities at discreet time points (as opposed to more frequent or continuous sampling over time) imposes limits on our understanding of the shorter vs. longer-term patterns and processes influencing benthic community structure in the bay.
Sediment grain size (i.e., silt and sand) was an important predictor of community and functional structure in the bay (except functional composition) with silt explaining the largest proportion of the observed variation. Overall, fine sediments in the historical community were associated with highly tolerant small-bodied subsurface deposit feeders such as C. pygodactylata, and Dorvilleidae spp. which contributed to ~ 11% of the dissimilarity between the time points. In comparison coarser sediments in the contemporary community contained a relatively higher proportion of small-medium (or medium-sized), surface deposit feeders (or suspension feeders) having pelagic planktotrophic or lecithotrophic larvae of which three bivalves (i.e., Nuculana pernula, Macoma calcarea, and Thyasira sp.) contributed ~ 7% to dissimilarity. Similarly, in the Wadden Sea Gusmao et al. (2022) examining functional traits along a sediment gradient found that coarser sediments were dominated by surface modifiers, suspension-feeding organisms, with larger bodies, whereas fine sediments associated with higher levels of organic matter were dominated by deposit feeders with small bodies. Liu et al. (2019) also found functional traits to vary with sediment type in the Arctic Bering Sea where silty-sandy sediments contained tube-dweller/burrower, deposit-feeding modalities, compared to sandy sediments predominantly containing motile taxa. Moreover, shifts in grain size as found in our present study could influence sediment geochemistry and may be important in influencing changes in macrofaunal abundance between time points. For instance, Clements and Hunt et al. (2018b) studying the clam Mya arenaria in the Bay of Fundy found that sites with larger grain size (i.e., coarser sediments) had a greater mean sediment pH, and mean clam abundance and sediment pH were positively correlated. McGarringle and Hunt (2024) also showed that sediment pH was the variable that best explained the variance in the multivariate community composition in the Bay of Fundy.
In addition to changes in sediment grain size, there has also been an increase in sedimentary TOM from the historical to contemporary times (i.e., 10.4 ± 0.52% vs. 13.5% ±0.98%, respectively). Though TOM is generally positively related to density (Davies and Payne 1984; Nestlerode et al. 2020), organic loading can decrease macrofaunal abundance (Pearson and Rosenberg 1978; Snelgrove and Butman 1994). In the present study, TOM was found to predict the density of macrofauna at both time points but was negatively related such that high levels of TOC were associated with lower densities. In the historical community, Ramey and Snelgrove (2003) attributed low macrofaunal densities to sulfide production in the sediments as well as poor food quality driven primarily by low densities at three sites that had the highest percentage of TOC. Similarly, darkened sediments (i.e., suggesting hydrogen sulfide accumulation) were seen during contemporary sample collection in both 2019 and 2020. Studies examining the effect of sediment organic carbon content on benthic communities in the Northern Hemisphere noted a range from 10 to 35 mg g−1 (which is equal to, TOM: 1.7–6.0%; TOC: 1.0–3.5%) as the general threshold where benthic communities would be expected to be reduced (Hyland et al. 2005; Walker et al. 2022).
While TOM in Placentia Bay sediments was also important in predicting species/taxon composition and abundance patterns in the bay it explained much less of the variability relative to grain size, and it was not an important predictor of functional structure. Interestingly, levels of TOM were elevated in the coarser contemporary sediments as compared to the finer sediments characterizing the historical time point (i.e., 10.4 ± 0.52% vs. 13.5% ±0.98%, respectively). As TOM is commonly associated with finer sediment grain sizes (Snelgrove and Butman 1994), this decoupling may be suggestive of possible effects of disturbance (e.g., storms and organic enrichment). Freshwater input during storm events, and/or from aquaculture in the bay, may be a source of organic enrichment (Haya et al. 2001; Bêche et al. 2006; Fuchs et al. 2020). Supply of organic matter can vary year-to-year, influenced by both freshwater inputs and climate/weather (Diaz and Rosenberg 1995). Placentia Bay experiences heavy rainfall during storm surges (e.g., ~ 200 mm hurricane Teddy, September 2020). It has freshwater inputs at the head of the bay including the Come by Chance River (mean discharge ~ 1.95 m3s−1) (MacIsaac et al. 2023) and Swift Current (Ramey et al. 2003). Moreover, sedimentary TOM has been shown to be highest at the head of the bay compared to other areas (Ramey et al. 2003; Komendić 2023). Changes in SST (Han et al. 2015) may also play a role as it can influence the timing and amount of food supply (e.g., primary production) to the benthos.
Overall, the reduced abundance of highly tolerant, deposit-feeding polychaetes in relatively coarser sediments characterizing the contemporary years, appears to support a more even distribution of functional traits related to tolerance, body size, feeding, bioturbation, and larval development which are important in resistance to disturbance, resources use, and energy flow (e.g., Rand et al. 2018; Sutton et al. 2020). Relatively high functional evenness has been suggested to support resilience through its relatively greater potential for the maintenance of ecosystem function (Sutton et al. 2020). Our findings suggest that communities have exhibited temporal stability or resilience with respect to functional traits despite the current levels of TOM.
Conclusion
Communities and habitats in Placentia Bay have undergone significant changes since 1998. Overall, the initial hypotheses were consistent with the results. Community and functional structure in the bay differed between historical and contemporary times and were influenced by the sedimentary habitat. Moreover, trait modalities were maintained between these time points (i.e., no loss of modalities), however, modalities were more evenly distributed in terms of the proportion of expressed traits in the contemporary community. Our findings suggest that communities have exhibited temporal stability or resilience with respect to functional traits despite the current levels of TOM; possibly through combined effects of high sediment TOC and increased grain size influencing the abundance of highly tolerant deposit-feeding polychaetes. Future monitoring in the bay should prioritize sampling to better quantify the extent of year-to-year seasonal variation of macrofauna in relation to the sedimentary habitat (i.e., sediment grain size, TOC, and C/N ratios) at critical time points including the summer and fall months. A long-term sampling station(s) that could be more frequently sampled would be useful in capturing shorter/longer-term changes/impacts associated with disturbances that may result from nearby aquaculture, temperature change, and sporadic events such as large inputs of rainfall, storm surges, and hurricanes.
References
Alexander, M. A., J. D. Scott, K. D. Friedland, K. E. Mills, J. A. Nye, A. J. Pershing, and A. C. Thomas. 2018. Projected Sea surface temperatures over the 21st century: changes in the mean, variability, and extremes for large marine ecosystem regions of Northern oceans. Elementa Science of the Anthropocene 6(9). https://doi.org/10.1525/elementa.191.
Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x.
Anderson, M.J. 2017. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. https://doi.org/10.1002/9781118445112.stat07841.
Anderson, M.J., R.N. Gorley, and K.R. Clarke. 2008. PERMANOVA + for primer: guide to software and statistical methods. Plymouth: PRIMER-E. https://learninghub.primer-e.com/books/permanova-for-primer-guide-to-software-and-statistical-methods.
Avila, L.A., S.R. Stewart, R. Berg, and A.B. Hagen. 2020. National hurricane center tropical cyclone report – hurricane Dorian. https://www.nhc.noaa.gov/data/tcr/AL052019_Dorian.pdf.
Balvanera, P., A. B. Pfisterer, N. Buchmann, J. S. He, D. Raffaelli, and B. Schmid. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters 9(10): 1146–1156. https://doi.org/10.1111/j.1461-0248.2006.00963.x.
Barry, J. P., and P. K. Dayton. 1991. Physical heterogeneity and the organization of marine communities. Chapter 14 in ecological heterogeneity, eds. J. Kolasa, and S. T. A. Pickett. 269–320. New York: Springer-. https://doi.org/10.1007/978-1-4612-3062-5_14.
Bêche, L. A., E. P. McElravy, and V. H. Resh. 2006. Long-term seasonal variation in the biological traits of benthic-macroinvertebrates in two Mediterranean-climate streams in California, U.S.A. Freshwater Biology 51: 56–75. https://doi.org/10.1111/j.1365-2427.2005.01473.x
Belley, R., and P. V. R. Snelgrove. 2016. Relative contributions of biodiversity and environment to benthic ecosystem functioning. Frontiers in Marine Science 3: 242. https://doi.org/10.3389/fmars.2016.00242.
Bengtsson, L., K. I. Hodges, and E. Roeckner. 2006. Storm tracks and climate change. Journal of Climate 19: 3518–3543. https://doi.org/10.1175/JCLI3815.1.
Beukema, J. J. 1976. Biomass and species richness of the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. Netherlands Journal of Sea Research 10(2): 236–261. https://doi.org/10.1016/0077-7579(76)90017-X.
Biggs, C. R., L. A. Yeager, D. G. Bolser, C. Bonsell, A. M. Dichiera, Z. Hou, S. R. Keyser, A. J. Khursigara, K. Lu, A. F. Muth, Jr. B. Negrete, and B. E. Erisman. 2020. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 11(7): e03184. https://doi.org/10.1002/ecs2.3184.
Borja, A., J. Franco., and V. Pérez. 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin 40: 1100–1114. https://doi.org/10.1016/S0025-326X(00)00061-8.
Bradbury, I. R., P. V. R. Snelgrove, and S. Fraser. 2000. Transport and development of eggs and larvae of Atlantic Cod, Gadus morhua, in relation to spawning time and location in coastal Newfoundland. Canadian Journal of Fisheries and Aquatic Sciences 57(9), 1761–1772. https://doi.org/10.1139/f00-122.
Bremner, J., S. I. Rogers, and C. L. J. Frid. 2006. Methods for describing ecological functioning of marine benthic assemblages using biological trait analysis (BTA). Ecological Indicators 6: 609–622. https://doi.org/10.1016/j.ecolind.2005.08.026.
Cadotte, M. W., K. Carscadden, and N. Mirotchnick. 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. Journal of Applied Ecology 48(5): 1079–1087. https://doi.org/10.1111/j.1365-2664.2011.02048.x.
Cangialosi, J. P. 2021. National hurricane center tropical cyclone report (Tropical storm Odette). National Hurricane Center. https://www.nhc.noaa.gov/data/tcr/AL152021_Odette.pdf.
Cardinale, B. J., E. Duffy, A. Gonzalez, D. U. Hooper, C. Perrings, P. Venail, A. Narwani, G. M. Mace, D. Tilman, D. A. Wardle, A. P. Kinzig, G. C. Daily, M. Loreau, J. B. Grace, A. Larigauderie, D. S. Srivastava, and S. Naeem. 2012. Biodiversity loss and its impacts on humanity. Nature 486: 59–67. https://doi.org/10.1038/nature11148.
CBC News. 2020. Placentia hit with 200 mm of rain in first significant storm of season. https://www.cbc.ca/news/canada/newfoundland-labrador/rain-burin-eastern-newfoundland-1.5731040. Accessed Mar 2024.
Center for Climate and C2ES, and Energy Solutions. 2020. Hurricanes and climate change. https://www.c2es.org/content/hurricanes-and-climatechange/#:~:text=More%20recently%20(2000%2D2014),partially%20related%20to%20global%20warming. Accessed 22 Apr 2022.
Chen, E. Y. S. 2021. Often overlooked: understanding and meeting the current challenges of marine invertebrate conservation. Frontiers in Marine Science 8: e690704. https://doi.org/10.3389/fmars.2021.690704.
Chen, Z., Y. Kwon, K. Chen, P. Frantantoni, G. Gawarskiewicz, and T. M. Joyce. 2020. Long-term SST variability on the Northwest Atlantic continental shelf and slope. Geophysical Research Letters. https://doi.org/10.1029/2019GL085455.
Chevenet, F., S. Dolédec, and D. Chessel. 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshwater Biology 31(3): 295–309. https://doi.org/10.1111/j.1365-2427.1994.tb01742.x.
Clarke, K. R. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x.
Clarke, K. R., and R. N. Gorley. 2015. PERMANOVA + primer V7: user Manual. Plymouth, UK: Prim. Ltd.
Clarke, K. R., R. N. Gorley, P. J. Sommerfield, and R. M. Warwick. 2014. Change in marine communities: an approach to statistical analysis. 3rd edition. PRIMER-E: Plymouth.
Clements, J., and H. L. Hunt. 2018a. Effects of CO2-driven sediment acidification on infaunal marine bivalves: a synthesis. Marine Pollution Bulletin 117: 6–16. https://doi.org/10.1016/j.marpolbul.2017.01.053.
Clements, J., and H. L. Hunt. 2018b. Testing for Sediment Acidification effects on within-season variability in Juvenile Soft-Shell clam (Mya arenaria) abundance on the Northern Shore of the Bay of Fundy. Estuaries and Coasts 41: 471–483. https://doi.org/10.1007/s12237-017-0270-x.
Covich, A. P., M. C. Austen, F. Bärlocher, E. Chauvet, B. J. Cardinale, C. L. Biles, P. Inchausti, O. Dangles, M. Solan, M. O. Gessner, B. Statzner, and B. Moss. 2004. The role of biodiversity in the functioning of freshwater and marine benthic ecosystems. BioScience 54(8): 767–775. https://doi.org/10.1641/0006-3568(2004)054[0767:TROBIT]2.0.CO;2.
Covich, A. P., M. A. Palmer, and T. A. Crowl. 1999. The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. BioScience 49(2): 119–127. https://doi.org/10.2307/1313537.
Danovaro, R. 2009. Chapter 1. In Total Organic Matter and Water Contents, grain size, Bulk Density, Porosity, and Redox Potential of Sediments. Methods for the study of deep-sea sediments, their functioning and Biodiversity. CRC.
Davies, J. M., and R. Payne. 1984. Supply of organic matter to the sediment in the northern North Sea during a spring phytoplankton bloom. Marine Biology 78: 315–324. https://doi.org/10.1007/BF00393017.
Diaz, R.J., and R. Rosenberg. 1995. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology, An Annual Review 33: 245–303.
Drejou, E., N. Desroy, J. Carrière, L. Tréau, C. W. de Coeli, McKindsey, and P. Archambault. 2021. Determining the ecological status of benthic coastal communities: a case in an anthropized sub-arctic area. Frontiers in Marine Science 8: 637546. https://doi.org/10.3389/fmars.2021.637546.
Dyer, K. R. 1986. Coastal and estuarine sediment dynamics. Wiley, Chichester.
Frid, C. L. J., and B. A. Caswell. 2015. Is long-term ecological functioning stable: the case of the marine benthos? Journal of Sea Research 98: 15–23. https://doi.org/10.1016/j.seares.2014.08.003.
Fuchs, H. L., R. J. Chant, E. J. Hunter, E. N. Curchitser, G. P. Gerbi, and E. Y. Chen. 2020. Wrong-way, migrations of benthic species driven by ocean warming and larval transport. Nature Climate Change 10: 1052–1056. https://doi.org/10.1038/s41558-020-0894-x.
Gallagher, E. D., and J. F. Grassle. 1997. Virginian Province macroinfaunal community structure: PCA-H analyses and an assessment of pollution degradation indices. 130. Rhode Island: Final Report to Environmental Protection Agency, Atlantic Ecology Division, Narragansett.
Gusmao, J. B., D. W. Theiltges, R. Dekker, L. L. Govers, K. J. Meijer, and B. K. Eriksson. 2022. Comparing taxonomic and functional trait diversity in marine macrozoobenthis along sediment texture gradients. Ecological Indicators 145: 109718. https://doi.org/10.1016/j.ecolind.2022.109718.
Hale, S. S., H. W. Buffum, J. A. Kiddon, and M. M. Hughes. 2017. Subtidal benthic invertebrates shifting northward along the US Atlantic Coast. Estuaries and Coasts 40: 1744–1756.
Han, G., E. B. Colbourne, P. Pepin, and Y. Xie. 2015. Statistical projections of ocean climate indices off Newfoundland and Labrador. Atmosphere– Ocean 53(3): 556–570. https://doi.org/10.1080/07055900.2015.1047732.
Harris, E. K., P. A. Montagna, A. R. Douglas, L. Vitale, and D. Buzan. 2023. Influence of an industrial discharge on long-term dynamics of abiotic and biotic resources in Lavaca Bay, Texas, USA. Environmental Monitoring and Assessment 195(1): 40. https://doi.org/10.1007/s10661-022-10665-w.
Haya, K., L. E. Burridge, and B. D. Chang. 2001. Environmental impact of chemical wastes produced by the salmon aquaculture industry. ICES Journal of Marine Science 58: 492–496. https://doi.org/10.1006/jmsc.2000.1034.
Herder, E. C., A. Aitken, and E. Edinger. 2021. Temporal change in the molluscan assemblages (bivalves and gastropods) of Frobisher Bay, Nunavut, Canada, over 50 years. Arctic Science 8: 200–227. https://doi.org/10.1139/as-2020-0037.
Herkül, K., K. Torn., U. Suursaar., V. Alari., and A. Peterson. 2016. Variability of benthic communities in relation to hydrodynamic conditions in the north-eastern Baltic Sea. Journal of Coastal Research 75: 867–871. https://doi.org/10.2112/SI75-174.1.
Hyland, J., L. Balthis, I. Karakassis, P. Magni, A. Petrob, J. Shine, O. Vestergaard, and R. Warwick. 2005. Organic carbon content of sediments as an indicator of stress in the marine benthos. Marine Ecology Progress Series 295: 91–103. https://doi.org/10.3354/meps295091.
JASP Team. 2022. JASP (Version 0.16) [Computer software]. https://jasp-stats.org/team/.
Kauppi, L., J. Norkko, J. Ikonen, and A. Norkko. 2017. Seasonal variability in ecosystem functions: quantifying the contribution of invasive species to nutrient cycling in coastal ecosystems. Marine Ecology Progress Series 572: 193–207. https://doi.org/10.3354/meps12171.
Kiceniuk, J. W. 1992. Aromatic hydrocarbon concentrations in sediments of Placentia Bay, Newfoundland. Canadian Technical Report of Fisheries and Aquatic Sciences No. 1888.
Komendić, I. 2023. Comparing historical and contemporary macrofaunal communities and functional traits in a subarctic embayment in Newfoundland, Canada. Winnipeg, Manitoba: Master of Science, University of Manitoba.
Legendre, P., and M. J. Anderson. 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69(1): 1–24. https://doi.org/10.1890/0012-9615(1999)069.
Legendre, P., and L. Legendre. 2012. Numerical ecology. Elsevier.
LGL. 2018. Fish and fish habitat. component study for the environmental impact statement of the Placentia Bay Atlantic Salmon Aquaculture Project. Report no. FA0144-2, 71 p. + appendices. https://www.gov.nl.ca/ecc/files/env-assessment-projects-y2016-1834-1834-eis-docs-cs-fish-and-fish-habitat.pdf. Accessed March 1 2022.
Lima, F. P., and D. S. Wethey. 2012. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nature Communications 3(1): 704. https://doi.org/10.1038/ncomms1713.
Liu, H., H. Xuebae, H. Yaqin, L. Zhong, L. Junhui, M. Jianfeng, Z. Shuy, L. Longshan, W. Jianjun, and S. Jun. 2019. Functional trait composition and diversity patterns of marine macrobenthos across the Arctic Bering Sea. Ecological Indicators 102: 673–685. https://doi.org/10.1016/j.ecolind.2019.03.029.
Ma, Z., G. Han, and B. DeYoung. 2012. Modeling temperature, currents, and stratification in Placentia Bay. Atmosphere-Ocean 50 (3): 244–260. https://doi.org/10.1080/07055900.2012.677413.
Ma, Z., G. Han, and B. DeYoung. 2017. Modeling the response of Placentia Bay to hurricanes Igor and Leslie. Ocean Modelling 112: 112–124. https://doi.org/10.1016/j.ocemod.2017.03.002.
MacIsaac, B. I., T. L. King, and A. C. Ortmann. 2023. State of Knowledge on Fate and Behaviour of Ship-Source Petroleum Product Spills: Volume 7, St. John’s & Placentia Bay, Newfoundland & Labrador. Canadian Manuscript Report of Fisheries and Aquatic Sciences 3253: vii + 32 p.
Mallin, M. A., M. H. Posey, M. R. McIver, D. C. Parsons, S. H. Ensign, and T. D. Alphin. 2002. Impacts and recovery from multiple hurricanes in a Piedmont – Coastal Plain River System: human development of floodplains greatly compounds the impacts of hurricanes on water quality and aquatic life. Bioscience 52: 999–1010. https://doi.org/10.1641/0006-3568(2002)052[0999:IARFMH]2.0.CO;2.
Manchia, C. M., R. P. Mulligan, D. J. Mallinson, and S. J. Culver. 2023. Coastal Response to the Landfall of a Hurricane on a series of inlets and narrow back-barrier waterways. Estuaries and Coasts 46: 1690–1708. https://doi.org/10.1007/s12237-023-01242-6.
Martini, S., L. F. Larras, A. Boyé, E. Faure, N. Aberle, P. Archambault, L. Bacouillard, B. E. Beisner, L. Bittner, E. Castella, M. Danger, O. Gauthier, L. Karp-Boss, F. Lombard, F. Maps, L. Stemmann, E. Thiébault, P. Usseglio-Polatera, M. Vogt, M. Laviale, and S. D. Ayata. 2020. Functional trait-based approaches as a common framework for aquatic ecologists. Limnology and Oceanography 66: 965–994. https://doi.org/10.1002/lno.11655.
Mazurkiewicz, M., B. Górska, P. E. Renaud, J. Legeżyńska, and J. Berge. and M. Włodarska-Kowalczuk1. 2019. Seasonal constancy (summer vs. winter) of benthic size spectra in an Arctic fjord. Polar Biology 42: 1255–1270. https://doi.org/10.1007/s00300-019-02515-2.
McGarrigle, S. A., and H. L. Hunt. 2024. Infaunal invertebrate community relationships to water column and sediment abiotic conditions. Marine Biology 171: 3. https://doi.org/10.1007/s00227-023-04318-w.
McLean, M., A. Auber, N. A. J. Graham, P. Houk, S. Villeger, C. Violle, W. Thuiller, S. K. Wilson, and D. Mouillot. 2019. Trait structure and redundancy determine sensitivity to disturbance in marine fish communities. Global Change Biology 25: 3424–3437. https://doi.org/10.1111/gcb.14662.
Méndez-Tejeda, R., and J. J. Hernández-Ayala. 2023. Links between climate change and hurricanes in the North Atlantic. PLoS Climate 2(4): e0000186. https://doi.org/10.1371/journal.pclm.0000186.
Montagna, P. A. 2023. Benthic infauna are resistant and resilient to hurricane disturbance. Marine Ecology Progress Series 707: 1–13. https://doi.org/10.3354/meps14263.
Morrisey, D. J., A. J. Underwood, L. Howitt. L., and J. S. Stark. 1992. Spatial variation in soft-sediment benthos. Marine Ecology Progress Series 81: 197–204. https://doi.org/10.1016/0022-0981(92)90177-C.
Muntadas, A. J., and M. Demestre. 2016. Assessing functional redundancy in chronically trawled benthic communities. Ecological Indicators 61: 882–892. https://doi.org/10.1016/j.ecolind.2015.10.041.
Nelson, D. W., and L. E. Sommers. 1996. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis (eds D.L. Sparks, A.L. Page, P.A. Helmke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C.T. Johnston and M.E. Sumner). https://doi.org/10.2136/sssabookser5.3.c34.
Nestlerode, J. A., M. C. Murrell, J. D. Hagy, L. Harwell, and J. A. Lisa. 2020. Bioassessment of a Northwest Florida estuary using benthic macroinvertebrates. Integrated Environmental Assessment and Management 16(2): 245–256. https://doi.org/10.1002/ieam.4209.
Olafsson, E. B., C. H. Peterson, and W. G. Ambrose. 1994. Does recruitment limitation structure populations and communities of macro-invertebrates in marine soft sediments: the relative significance of pre- and post- settlement processes. Oceanography and Marine Biology Annual Review: An Annual Review 32: 65–109.
Orton, P. M., T. M. Hall, S. A. Talke, A. F. Blumberg, N. Georgas, and S. Vinogradov. 2016. A validated tropical-extratropical flood hazard assessment for New York Harbor. Journal of Geophysical Research: Oceans 121: 8904–8929. https://doi.org/10.1002/2016JC011679.
Oug, E., A. Fleddum, B. Rygg, and F. Olsgard. 2012. Biological traits analyses in the study of pollution gradients and ecological functioning of marine soft bottom species assemblages in a fjord ecosystem. Journal of Experimental Marine Biology and Ecology 432–433: 94–105. https://doi.org/10.1016/j.jembe.2012.07.019.
Pearson, T. H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology 16: 229–311.
Podani, J., and I. Miklós. 2002. Resemblance coefficients and the horseshoe effect in principal coordinates analysis. Ecology 83: 3331–3343. https://doi.org/10.1890/0012-9658.
Pribyl, D. W. 2010. A critical review of the SOC to SOM conversion factor. Geoderma 156(3–4): 75–83.
Ramette, A. 2007. Multivariate analyses in microbial ecology. FEMS Microbiology Ecology 62(2): 145–160. https://doi.org/10.1111/j.1574-6941.2007.00375.
Ramey, P. A. 2001. Factors influencing patterns in distribution, abundance, and diversity of sedimentary macrofauna. In Deep, muddy sediments of Placentia Bay, Newfoundland, and the adjacent shelf, Master of Science, Memorial University of Newfoundland, St. John’s Newfoundland.
Ramey, P. A. 2011. Benthic index development: Assessment of ecological status of benthic communities in New Jersey marine coastal waters. Technical Report to the U.S. Environmental agency and new jersey department of environmental protection, institute of marine and coastal sciences, Rutgers University, New Jersey-United States 1–192.
Ramey, P. A., and E. Bodnar. 2008. Active post-settlement selection by a deposit-feeding polychaete Polygordius Jouinae, for sands with relatively high organic content. Limnology and Oceanography 53(4): 512–520. https://doi.org/10.2307/40058271.
Ramey, P. A., and P. V. R. Snelgrove. 2003. Spatial patterns in sedimentary macrofaunal communities on the south coast of Newfoundland in relation to surface oceanography and sediment characteristics. Marine Ecology Progress Series 262: 215–227. https://doi.org/10.3354/meps262215.
Ramón, M., E. Marco-Herrero, E. Galimany, L. Recasens, and P. Abello. 2023. Faunistic and structural changes in shallow benthic communities of the Ebre Delta (NW Mediterranean Sea). Diversity 15(5): 623. https://doi.org/10.3390/d15050623.
Rand, K., E. Logerwell, B. Bluhm, H. Chenelot, S. Danielson, K. Iken, and L. Sousa. 2018. Using biological traits and environmental variables to characterize two arctic epibenthic invertebrate communities in and adjacent to Barrow Canyon. Deep Sea Research Part II: Topical Studies in Oceanography 152: 154–169. https://doi.org/10.1016/j.dsr2.2017.07.015.
Rasch, D., F. Tuescher, and V. Guird. 2007. How robust are tests for two independent samples? Journal of Statistical Planning and Inference 173: 2706–2720. https://doi.org/10.1016/j.jspi.2006.04.011.
Reguero, B. G., L. J. Losada, and F. J. Méndez. 2019. A recent increase in global wave power as a consequence of oceanic warming. Nature Communications 10(1): 205. https://doi.org/10.1038/s41467-018-08066-0.
Reiss, H., and I. Kroncke. 2005. Seasonal variability of Benthic indices: an Approach to test the applicability of different indices for Ecosystem Quality Assessment. Marine Pollution Bulletin 50: 1490–1499. https://doi.org/10.1016/j.marpolbul.2005.06.017.
Renaud, P. E., M. Wtodarska-Kowalczuk, H. Trannum, B. Holte, J. M. Wgstawski, S. Cochrane, D. Salve, and B. Gulliksen. 2007. Multidecadal stability of benthic community structure in a high-Arctic glacial fjord (van Mijenfjord, Spitsbergen). Polar Biology 30(3): 295–305. https://doi.org/10.1007/s00300-006-0183-9.
Reynoldson, T. B. 1984. Interactions between sediment contaminants and benthic organisms. Hydrobiologia 149: 53–66. https://doi.org/10.1007/BF00048646.
Rhoads, D. C., and D. K. Young. 1970. The influence of deposit-feeding organisms on sediment stability and community trophic structure. Journal of Marine Research 28(2): 150–178. https://doi.org/10.1357/002224020834162167.
Snelgrove, P. V. R., and C. A. Butman. 1994. Animal sediment relationship revisited – cause Versus Effect. Oceanography and Marine Biology 32: 111–177.
Snelgrove, P. V. R., K. Soetaert, M. Solan, M. Solan, S. Thrush, C. L. Wei, R. Danovero, R. W. Fulweiler, H. Kitazato, B. Ingole, A. Norkko, R. J. Parkes, and N. Volkenborn. 2018. Global carbon cycling on a heterogenous seafloor. Trends in Ecology and Ecology 33(2): 96–105. https://doi.org/10.1016/j.tree.2017.11.004.
Sutton, L., K. Iken, B. A. Bluhm, and F. J. Mueter. 2020. Comparison of functional diversity of two alaskan Arctic shelf epibenthic communities. Marine Ecology Progress Series 651: 1–20. https://doi.org/10.3354/meps13478.
Taghon, G. L., P. A. Ramey, C. M. Fuller, R. F. Petrecca, and J. P. Grassle. 2017b. Benthic community structure and sediment properties in Barnegat Bay, New Jersey, before and after Hurricane Sandy. Estuaries and Coasts 40: 160–172. https://doi.org/10.1007/s12237-016-0133-x.
Taghon, G. L., P. A. Ramey, C. M. Fuller, R. F. Petrecca, J. P. Grassle, and T. J. Belton. 2017a. Benthic invertebrate community composition and sediment properties in Barnegat Bay, New Jersey, 1965–2014. Journal of Coastal Research 78: 169–183. https://doi.org/10.2112/SI78-013.1.
Valderhaug, V. A., and J. S. Gray. 1984. Stable macrofauna community structure despite fluctuating food supply in subtidal soft sediments of Oslofjord, Norway. Marine Biology 82: 307–322. https://doi.org/10.1007/BF00392411.
Van Geest, J. L., K. A. Kidd, H. L. Hunt, M. J. Abgrall, M. J. Maltais, and A. Mercer. 2015. Development of baseline data for long-term monitoring of sediment conditions at reference sites in Saint John Harbour, New Brunswick: benthic infaunal invertebrates and sediment contaminants 2011–2013. Canadian Manuscript Report of Fisheries and Aquatic Sciences 3076: 97.
Van Hoey, G., A. Borja, S. Birchenough, L. Buhl-Mortensen, S. Degraer, D. Fleischer, F. Kerckhof, P. Magni, I. Muxika, H. Reiss, A. Schroder, and M. L. Zettler. 2010. The use of benthic indicators in Europe: from the Water Framework Directive to the Marine Strategy Framework Directive. Marine Pollution Bulletin 60: 2187–2196. https://doi.org/10.1016/j.marpolbul.2010.09.015.
Van Ruth, P. D., G. G. Ganf, and T. M. Ward. 2010. The influence of mixing on primary productivity: a unique application of classical critical depth theory. Progress in Oceanography 85: 224–235. https://doi.org/10.1016/j.pocean.2010.03.002.
Vaquer-Sunyer, R., and C. M. Duarte. 2010. Sulfide exposure accelerates hypoxia-driven mortality. Limnology Oceanography 55: 1075–1082. https://doi.org/10.4319/lo.2010.55.3.1075.
Vause, B. J., S. A. Morley, V. G. Fonseca, A. Jazdzewska, G. V. Ashton, D. K. A. Barnes, H. Giebner, M. S. Clark, and L. S. Peck. 2019. Spatial and temporal dynamics of Antarctic shallow soft-bottom benthic communities: ecological drivers under climate change. BMC Ecology 19: 27. https://doi.org/10.1186/s12898-019-0244-x.
Walker, B. H. 1992. Biodiversity and ecological redundancy. Conservation Biology 6(1): 18–23.
Walker, J. B., D. J. Gillett, and M. Sutula. 2022. Establishing biologically relevant sediment organic matter threshold for estuaries and embayments of the Southern California Bight, USA. Ecological Indicators 143. https://doi.org/10.1016/j.ecolind.2022.109404.
Wei, T., and V. Simko. 2017. R package corrplot: visualization of a correlation Matrix (Version 0.84). https://cran.r-project.org/web/packages/corrplot/corrplot.pdf.
Weissberger, E. J., P. A. Jumars, L. M. Mayer, and L. L. Schick. 2008. Structure of a northwest Atlantic Shelf macrofaunal assemblage with respect to seasonal variation in sediment nutritional quality. Journal of Sea Research 60: 164–175.
Wells, N., K. Tucker, K. Allard, M. Warren, S. Olson, L. Gullage, C. Pretty, V. Sutton-Pande, and K. Clarke. 2019. Re-evaluation of the placentia bay-grand banks area of the newfoundland and labrador shelves bioregion to identify and describe ecologically and biologically significant areas. DFO Canadian Science Advisory Secretariat Research Documents viii + 151
Xu, G., G. Han, C. Dong, J. Yang, and B. DeYoung. 2019. Observing and modeling the response of Placentia Bay to an extratropical cyclone. Atmosphere 10(11): 724. https://doi.org/10.4319/lo.2010.55.3.1075.
Acknowledgements
We would like to thank the Benthic Ecology team (DFO-NL) and colleagues for the collection of the field samples in 2019–2022 and/or sample processing (K. Bussey, R. Deering, R. Dove, L. Freeman, V. Hayes, P. Hawkins, E. Herder, C. Malayny, B. Piercey, and A. Robar), as well as Burin Tradition’s captain W. Pitcher and L. Fudge (vessel crew). Contemporary macrofaunal samples were identified by L. Treau-De Coli (Université Laval, Quebec). Dr. G. Davoren provided constructive comments on earlier drafts of the manuscript along with two reviewers. The research was supported by the Placentia Bay Coastal Environmental Baseline Program (DFO-NL, Canada) and associated colleagues K. Dalley, L. Freeman, J. Higdon, and K. Tucker. This project was partially funded by Fisheries and Oceans Canada’s Coastal Environmental Baseline Program (Placentia Bay, Newfoundland and Labrador Region).
Funding
This project was partially funded by Fisheries and Oceans, Canada’s Coastal Environmental Baseline Program (Placentia Bay, Newfoundland and Labrador Region).
Author information
Authors and Affiliations
Contributions
All authors listed, significantly contributed to the manuscript. BN designed and led the sampling program (with input from PRB). BN developed the overarching goals. All authors contributed to the initial examination and quality control of the data. IK and PRB conducted the statistical analysis. IK drafted the first version of the manuscript under the supervision of PRB. All authors contributed to subsequent writing and editing. All authors have approved the submission of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The last author Patricia Ramey-Balci is an Associate Editor for Estuaries and Coasts.
Disclaimer
This manuscript details original research that has not been previously published, nor is it under consideration for publication elsewhere. All figures, tables, and text passages have never been published elsewhere but have been uploaded to the pre-print server bioRxiv prior to submission to Estuaries and Coasts. The preprint will be updated with the publication reference, including the DOI and a URL link to the published version of the article on the bioRxiv website.
Additional information
Communicated by John Carroll
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Komendić, I., de Moura Neves, B. & Ramey-Balci, P.A. What has Changed in 20 Years? Structure and Function of Soft-sediment Macrofauna in a Subarctic Embayment, Newfoundland (Canada). Estuaries and Coasts (2024). https://doi.org/10.1007/s12237-024-01377-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-024-01377-0