Abstract
Fish use of saltmarsh varies spatially, temporally, and with environmental conditions. The specific impact of these effects on fish assemblages in southern temperate Tasmania, Australia—the only mangrove-free Australian state—is as yet largely unknown. Seasonal variation in fish abundance, richness, diversity, and size was investigated in succulent saltmarshes in three estuaries (Marion Bay, Barilla Bay, and Ralphs Bay) in south-eastern Tasmania. All parameters varied between sampling locations. Greater numbers of fish were recorded at two sites (Marion Bay, mean density and standard error of 396.9 ± 71.3 individuals per 100 m2; Barilla Bay, mean density and standard error of 94.1 ± 30.1 individuals per 100 m2) than have been previously reported in Australian saltmarshes. Fish abundance was greatest in July–August (mean density and standard error of 200.2 ± 49.7 individuals per 100 m2) reflecting the breeding patterns of the numerically dominant Atherinosoma microstoma. Both abundance and species richness responded positively to water temperature in ordinal logistic regression models, and species richness and diversity increased with water depth in the models. It is likely that the strong differences between sampling locations are partly related to differences in water depth and water temperature between the estuaries. They may be also related to the habitat context of each estuary, especially the presence or absence of seagrass. The greater numbers of fish found in the present study relative to abundances reported in mainland Australia may relate to the absence of mangroves and the consequent differences in seascape habitat context, including greater water depths in marshes. Importantly, these results demonstrate that temperate southern hemisphere saltmarshes are year-round habitat for fish, thus emphasising their importance as a fish habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal saltmarshes provide nursery (Hettler 1989; Kneib 1997; França et al. 2009), feeding (West and Zedler 2000; Crinall and Hindell 2004; Platell and Freewater 2009; Mazumder et al. 2011), and refuge (Boesch and Turner 1984; Warren et al. 2001; Odum 2002) habitat for fish. The use of saltmarsh habitat by fish varies between regions and geographical contexts (Ziegler et al. 2021), with differences in fish abundance and diversity observed at small to large spatial and temporal scales (Thomas and Connolly 2001; Mazumder et al. 2006b; Rountree and Able 2007; Ennis and Peterson 2015; Prahalad et al. 2019). These spatio-temporal patterns in variation of fish use of saltmarshes have been viewed as being hierarchical, with nested temporal, spatial, biotic, and abiotic contexts situated within larger regional settings (Bradley et al. 2020).
Variation in fish use of saltmarshes has been associated with differences in flooding patterns (also known as hydroperiod, incorporating inundation variables depth, frequency and duration; Rozas 1995) and seascape context (e.g. proximity to other neighbouring habitat types; Baillie et al. 2015), and may vary with latitude and climate (Mariano-Jelicich et al. 2014; Prahalad et al. 2019; Ziegler et al. 2021). In the USA, for example, marsh flooding patterns vary substantially across the Gulf and Atlantic coasts, influencing access by fish (Rozas 1995). Positive correlations have also been found between flooding duration and the magnitude of the trophic transfer between producers and consumers (Baker et al. 2013). Seascape context, particularly the presence or absence of particular subtidal and intertidal habitats, is also an important influence on fish. At a continental scale, there are latitudinal trends in assemblage structure and species diversity in Europe (França et al. 2009), South America (Mariano-Jelicich et al. 2014), and Australia (Prahalad et al. 2019), with greater diversity found at higher latitudes.
Within individual saltmarshes, flooding patterns (particularly depth; Thomas and Connolly 2001; Prahalad et al. 2019), microtopography, and distance from the marsh edge can influence variation in fish use (Baltz et al. 1993; Peterson and Turner 1994; Osgood et al. 2003). Species richness (Thomas and Connolly 2001) and fish density (Osgood et al. 2003) are positively correlated with water depth. Both transient and resident species utilise deeper water close to the marsh edge, while resident species are found in the marsh interior (Peterson and Turner 1994). Some juvenile residents remain in shallow microhabitats at low tide, thus avoiding piscivorous predation (Kneib 1987). Changes in microtopography associated with invasive plant species reduce this nursery function where tidal flooding frequency is affected (Osgood et al. 2003). Similarly, where plant invasions of tall grassy species into low succulent vegetation affect fish movement and habitat accessibility, fish abundance and diversity are lower (Harrison‐Day et al. 2022).
Diel and seasonal variation can also influence habitats for marsh fish (Rountree and Able 2007), although in some cases, where diel and seasonal tidal patterns coincide, their individual effects can be difficult to separate (Thomas and Connolly 2001). Where diel patterns have been distinguished from seasonal patterns, however, higher abundances of frequently occurring species have been recorded at night, possibly due to reduced predation risk (West and Zedler 2000; Prahalad et al. 2019). Seasonal variation in fish use of saltmarsh (Talbot and Able 1984; Kneib 1991; Valiñas et al. 2012) is influenced by changes in biotic and abiotic conditions, including but not limited to temperature, photoperiod, salinity, food availability, predation, and competition (Rountree and Able 2007). Seasonal variation may be associated with the nursery and refuge values of the habitat (Rountree and Able 2007) or with the feeding opportunities provided by the seasonal availability of prey species (Mazumder et al. 2006a; McPhee et al. 2015).
In Australia, studies on fish use of vegetated saltmarsh flats have been carried out in subtropical and temperate waters. Densities of fish reported in Australian saltmarshes range from 0.4 fish per 100 m2 in a South Australian estuary (Bloomfield and Gillanders 2005) to 72.4 per 100 m2 in Tasmania (Prahalad et al. 2019). Reported species richness ranges from 23 species in a Queensland marsh (Thomas and Connolly 2001) to a single species in South Australia (Bloomfield and Gillanders 2005). A latitudinal trend in species richness along the Australian east coast has been suggested (Prahalad et al. 2019), with species richness decreasing from north to south. Other regional differences include the numerical dominance of the ambassid Ambassis jacksoniensis in lower temperate latitudes and the atherinid Atherinosoma microstoma in higher temperate latitudes (Mazumder et al. 2006b; Platell and Freewater 2009; Prahalad et al. 2019). Strong seasonal variation has yet to be observed in temperate Australian saltmarshes (Bloomfield and Gillanders 2005; Mazumder et al. 2005). Some seasonal variation in fish assemblages has been described in lower latitudes (Thomas and Connolly 2001; Connolly 2005) but since tidal inundation corresponds with night hours during winter and daytime in summer the influence of season is unclear.
Tidal and environmental conditions also differ across the Australian continent. Studies in mainland Australian saltmarshes report findings from microtidal to low mesotidal marshes (e.g. Bloomfield and Gillanders 2005; Connolly 2005; Mazumder et al. 2006b; Platell and Freewater 2009), where in many cases, inundation only occurs during spring high tides. In some instances, inundation only occurs when low atmospheric pressure events coincide with spring tides (Crinall and Hindell 2004). In other tidal contexts, such as the mesotidal estuaries of north-western Tasmania (which is south relative to mainland Australia), fish have been recorded during both spring and neap high tides, when marshes are fully and partially flooded (Prahalad et al. 2019).
Local habitat context, including composition of neighbouring habitat mosaics, and seascape configuration, is increasingly recognised to be an important influence on fish abundances in estuarine habitats (e.g. Bradley et al. 2019; Ziegler et al. 2021). Seascape habitat configuration varies across Australian regions. In the sub-tropical and low-temperature latitudes of mainland Australia, coastal saltmarshes are frequently situated behind mangroves, occupying higher intertidal elevations than the lower-lying mangroves (Saintilan et al. 2007; Kumbier et al. 2021). However, in the high-temperate southern island state of Tasmania, mangroves are absent (Kelleway et al. 2017). Fish abundances and, in some cases, species richness are higher in mangrove habitat than in adjacent saltmarsh (Bloomfield and Gillanders 2005; Mazumder et al. 2005). However, fish densities across saltmarsh and mangroves have been found to be similar when corrected for water volume (Bloomfield and Gillanders 2005; Mazumder et al. 2005).
In Australia, differences in assemblages are also evident between saltmarsh and seagrass habitats (Saintilan et al. 2007), with seagrass meadows supporting higher numbers of fish and greater species richness than saltmarsh (Bloomfield and Gillanders 2005). Although, fish have been recorded moving between saltmarsh and seagrass habitats with tidal flooding (Saintilan et al. 2007). Furthermore, saltmarshes that are highly connected with neighbouring seagrass have been found to have higher abundance and diversity than either saltmarsh or seagrass by themselves (Baillie et al. 2015).
In both Australia and elsewhere in the world, there is a need to extend our knowledge of the use and function of saltmarshes as fish habitat to inform conservation and restoration. In longer-studied saltmarsh contexts, such as the Gulf of Mexico and the Atlantic Coast of the USA, a body of research spanning several decades has provided extensive baseline knowledge of the functions and spatial and temporal variations in fish use of saltmarshes, on which restoration and management approaches have been built (e.g. Swamy et al. 2002; Warren et al. 2002). As awareness grows of geographic variation in saltmarshes (Ziegler et al. 2021) and the importance of habitat and environmental context in structuring fish assemblages (Bradley et al. 2019), location-specific information is needed on which to base sound management decisions. While this information is now available for parts of mainland Australia, it is still lacking in Tasmania.
Existing evidence from Tasmania suggests higher fish densities than elsewhere in the country (Prahalad et al. 2019; Harrison-Day et al. 2022), possibly related to the effects of the absence of mangroves. This work is limited to the meso-tidal context of northern Tasmania and the data are only from the summer months. Given fish habitat restoration projects are already being funded in the micro-tidal environments of south-eastern Tasmania, there is a need for strong baseline data that documents fish abundance and diversity, across diverse seascape settings, and throughout the year (Prahalad and Kirkpatrick et al. 2019). To address these gaps, the present study investigates the effects of location, time of year, and environmental conditions on fish assemblages, namely (a) fish abundance, (b) species richness, (c) species diversity, and (d) fish length, in microtidal south-eastern Tasmanian saltmarshes across four seasons. We anticipate the findings from this work will help develop better predictions and guidelines for saltmarsh conservation and restoration, both in Tasmania and in other similar contexts globally.
Methods
Site Selection and Characteristics
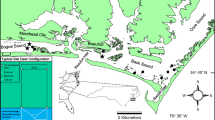
Three sampling locations were selected in south-east Tasmania (Fig. 1), at Barilla Bay (GDA94 MGA55 538856E, 5259700N), Ralphs Bay (GDA94 MGA55 539897E, 5247004N), and Marion Bay (GDA94 MGA55 570513E, 5258841N), based on desktop investigation and field reconnaissance.
Map showing locations of a Australian continent; b Tasmania; c study regions and the locations of the three study sites: Ralphs Bay, Barilla Bay, Marion Bay; d checking and resetting of buoyant pop nets at Ralphs Bay (Photograph: V Prahalad); e buoyant pop nets in action at Barilla Bay (Photograph: V Prahalad); and f top-down view of buoyant pop net at Marion Bay (Photograph: V. Harrison-Day). Coastal saltmarsh mapping data from Prahalad and Kirkpatrick (2019); SeaMap seagrass data from Lucieer (2007) and Ramsar data from Land Tasmania (2015), available from the LIST (www.thelist.tas.gov.au, Accessed 10 August 2022; State of Tasmania)
Each location had a vegetation structure and composition that is characteristic of the succulent saltmarshes in the region (Prahalad and Kirkpatrick 2019). There was little variation in the vegetation among sites targeted for sampling in the study locations. Marion Bay sampling sites were dominated by Sarcocornia quinqueflora with some Samolus repens, while Tecticornia arbuscula, Suaeda australis, and Hemichroa pentandra were also present. Less than 10% of the area enclosed by the sampling net was bare ground. Most sampling sites at Ralphs Bay were dominated by S. repens, with the remaining dominated by S. quinqueflora. T. arbuscula was present in one site (less than 5% cover), and all sampling sites contained bare ground, covering up to a third of the area enclosed by the net. Sites in Barilla Bay were dominated by S. quinqueflora and in some cases both S. quinqueflora and S. repens. No other plant species were recorded at Barilla Bay sampling sites and approximately half the enclosed area was covered by bare ground.
Estuarine geomorphic settings of the sites were two marine inlets (Pitt Water and Blackman Bay) and a drowned river valley (Derwent Estuary), as classified by Edgar et al. (1999). Tidal patterns in this region are semidiurnal with some diurnal inequality, and a microtidal range of up to 1.75 m (Bureau of Meteorology 2021b). Tidal flows on to marsh (depth, duration, frequency) are further modulated by geomorphic settings characteristic of each estuarine system and local topography (Palmer et al. 2019, Harrison-Day and Palmer unpublished data).
Nearest weather stations in south-east Tasmania record a mean annual rainfall of 495 mm (mean rainfall of 42.4 mm in December–February, 37.9 mm in March–May, 40.9 mm in June–August, and 43.9 mm in September–November; Bureau of Meteorology 2022). The mean daily maximum annual temperature for the area is 17.6 °C (with monthly mean daily maximum temperatures of 21.9 °C in December–January, 18.1 °C in March–May, 13.0 °C in June–August, and 15.5 °C in September–November; Bureau of Meteorology 2022).
The period of sampling (2020–2021) was characterised by above-average rainfall during the winter months, a drier-than-average November, and average to below-average rainfall for the remaining sampling months, with air temperatures mostly average to warmer than average (Bureau of Meteorology 2021a, 2022). The semi-diurnal tidal patterns ensure inundation of the vegetated saltmarsh flat usually occurs only once per approximately 24 h during spring high tide periods in the three sampling locations. During April–May and July–August sampling, the highest tides occurred at night. During November and January, the highest tides occurred in daylight.
There are human impacts on all three saltmarshes (Edgar et al. 2000; Prahalad and Jones 2013), with urban development (especially surrounding Hobart, Tasmania’s capital city), agricultural impacts, and climate change causing saltmarsh degradation and loss (Prahalad and Kirkpatrick et al. 2019; Visby and Prahalad 2020). Further details of the environment at each site can be found in Online Resource 1.
Data Collection
Sampling was carried out during spring high tides in July–August, November, January and April–May over 1 year (2020–2021). At each site, sampling occurred for two consecutive days on each of the four occasions, with four pop nets located at similar elevations in succulent saltmarsh and within 1–4 m of the marsh edge. Thus, there were eight net releases over two days at each site, with 24 releases across all sites per sampling round, and 96 releases in total during the year. The order of sampling location was alternated each sampling round so that the same location was not repeatedly sampled at the beginning or end of the spring tide period.
Pop nets have been frequently used in Australian studies investigating fish use of saltmarsh flats (e.g. Bloomfield and Gillanders 2005; Connolly 2005; Mazumder et al. 2005; Prahalad et al. 2019). They were chosen over other methods because of their provision of a repeatable and comparable measure of fish density per area and volume (Harrison-Day et al. 2020). Their large size (~ 25m2) mitigates problems associated with fine-scale local environmental heterogeneity (Harrison-Day et al. 2020).
The pop nets were 5 m × 5 m with 1-m-high walls and 2-mm mesh (see Online Resource 2 for details of their construction). Nets were installed at low tide on the dry marsh flats. The nets were released at slack high tide. Remote release (10–15 m) was achieved by two field personnel simultaneously pulling strings attached to the weights, freeing the flotation pipes at the top of the net walls and allowing them to rise to the surface within approximately one second. Fish contained within the net were then caught using hand nets as the water level receded. All net walls and small depressions inside the net were inspected for any remaining fish before the sampling effort was concluded. Nets remained in place for two consecutive days (reset for redeployment after the first sampling day) and were then removed. All nets and equipment were cleaned before further deployment at the next location to mitigate biosecurity risks associated with transport of sediment and any living material between sites.
Fish collected from the nets were identified, measured, and recorded in the field before being released. The total length (TL, tip of snout to tip of tail) of all individual fish was measured to the nearest 5 mm. Additional fish species opportunistically observed in the vicinity of the nets were also recorded based on in situ visual identification. Fish sampling and preservation necessary for identification followed University of Tasmania Animal Ethics Committee protocols (permit number A0018547).
Environmental variables generally associated with fish (Harrison-Day et al. 2020) were recorded from each site at each time of sampling. These were water depth, time of net release, diel phase (light or dark), water temperature, salinity, and pH. We included measurements of salinity and pH to account for potential freshwater influxes from overland flow or streams of variable pH present in marshes at the time of sampling. This proved not to be the case.
Water depth was measured as mean of maximum and minimum depths within each net at the time of net release, measured with a handheld ruler. Water temperatures were recorded at slack high tide (at the same time as pop net release) using a handheld thermometer. Water depth in south-eastern Tasmanian saltmarshes rarely exceeds 50 cm (Harrison-Day and Palmer unpublished data), and water is well mixed given the movement of tides. One measurement of temperature, and three water samples were therefore taken within 15 cm of the water surface, per sampling occasion. Salinity and pH were tested in a laboratory setting using the three water samples taken on each sampling occasion (tested three times per sample and averaged for reporting) using a precalibrated Hanna Instruments EC/TDS/Resistivity meter, model HI98192 (Hanna Instruments, USA) and a temperature compensated precalibrated Hanna Instruments pH meter, model 99,121 (Hanna Instruments, USA).
Data Analyses
To help interpret our predictive models and understand environmental variability between locations and times, we used analysis of variance (ANOVA) to relate each of water depth, water temperature, salinity, and pH between sampling months and between sampling locations. In the cases of pH and salinity, the analyses did not assume equality of variance. Chi-squared was used to test for independence between sampling seasons and diel phases. See Online Resource 1 for these results.
Ordinal logistic regression models were developed to investigate variation in fish abundance, species richness, and diversity (Shannon-Weiner H′) related to location (Marion Bay, Ralphs Bay, and Barilla Bay), month (July–August, November, January, and April–May) and environmental variables (water depth, water temperature, salinity, and pH). Fish abundance, species richness, and diversity data were each treated as ordinal class variables, with the classes established according to observed breaks in values (with groups containing at least eight values). This procedure was adopted because of multi-modality in the data. Abundance was grouped as class 0 = 0 individuals, 1 = 1–4, 2 = 5–13, 3 = 14–27, 4 = 28–54, 5 = 55–87, 6 = 88–151, and 7 ≥ 152. Species richness was grouped as class 0 = 0 species, 1 = 1, 2 = 2, and 3 = 3–4. Diversity was grouped as class 0 = 0 diversity (Shannon-Weiner H′ index), 1 = 0.040–0.188, 2 = 0.189–0.402, 3 = 0.403–0.636, and 4 ≥ 0.637. The ordinal logistic regression procedure, with default options in Minitab16 (Minitab Inc. 2010), was used to search for the most explanatory model in terms of concordant percent for each of the response variables in which all components had slopes with P < 0.05. The least explanatory variables were successively eliminated. All combinations of the final variables in the model were assessed to calculate the highest percentage concordance for a single variable and the highest addition to that concordance by the other variable or variables for each of the remaining variables. All mean values are reported with their standard error (SE). Box plots were constructed for independent variables that affected the dependent variables.
Non-metric multidimensional scaling (NMDS) ordination based on the Bray-Curtis dissimilarity measure (Clarke and Warwick 2001) with envfit and BIO-ENV routines were used to investigate environmental variables explaining fish community patterns. R (R Core Team 2019) and RStudio (Rstudio Team 2016) were used for these analyses. NMDS ordination was carried out using the function metaMDS, and environmental factors were fitted using envfit, with both functions from the vegan library (Oksanen et al. 2011). Stress levels of 0.20 in two dimensions were considered acceptable (Quinn and Keough 2002).
Mean fish length was calculated for the most abundant species, Atherinosoma microstoma, for each net release on each sampling occasion. A general linear model, using the default options in Minitab16, was used to investigate the effects of location, month, and environmental variables on A. microstoma mean length.
Results
Variation in Fish Abundance, Richness, Diversity, Community Patterns, and Length
Abundance
A total of 4112 fish were sampled in the 96 pop net releases. One hundred and eighty-four individuals (4.47% of total catch) were sampled at Ralphs Bay (with a mean density and standard error (SE) of 23 ± 8.6 (median 4.0) individuals per 100 m2), 753 at Barilla Bay (18.31%, mean 94.1 ± 30.1 (median 16.0) individuals per 100 m2), and 3175 at Marion Bay (77.21%, mean 396.9 ± 71.3 (median 282) individuals per 100 m2; Table 1).
Similar overall abundances occurred in July–August (1201 individuals, 29.21% of total catch, mean 200.2 ± 49.7 (median 94) individuals per 100 m2), November (1172 individuals, 28.51%, mean 195.3 ± 63.1 (median 38) individuals per 100 m2), and January (1117 individuals, 27.16%, mean 186.2 ± 88 (median 8) individuals per 100 m2; Table 2). Lower numbers of fish were sampled in April–May (622 individuals, 15.13%, mean 103.7 ± 31.7 (median 16) individuals per 100 m2).
The most explanatory ordinal logistic regression model for fish abundance consisted of location (57.4% concordance), water temperature which added 14.8% concordance, and sampling month which added 3.5% concordance (Table 3). In this model, the Marion Bay sampling location and July/August, November, and January sampling months had positive coefficients. Higher fish abundances were observed at Marion Bay, and with warmer water temperature (Fig. 2).
Fish abundance per net sample by explanatory variables: a sampling location (Barilla Bay, Marion Bay, Ralphs Bay) and sampling month (July/August, November, January, April/May), b water temperature (°C). Tone and shape indicate sampling location. Plot displays median line, lower and upper hinges (25th and 75th percentiles), upper and lower whiskers (extending to largest and smallest value no further than 1.5 times inter-quartile range), and outlying points
Richness
Seventeen fish species were recorded. Atherinids (almost exclusively A. microstoma) comprised 87% of individuals. The mullet Aldrichetta forsteri was the next most common species, representing 9.46% of the number of individuals sampled. The total number of species recorded was similar at Barilla Bay (11 species) and Marion Bay (10 species), although several species differed between the sites (Table 1). Only four species were recorded at Ralphs Bay, all of which were also found at other sites. Fishes that occurred in Barilla Bay and not in Marion Bay included the flounder Rhombosolea tapirina, atherinid Leptatherina presbyteroides, and Tasmanian smelt Retropinna tasmanica. Species that occurred at Marion Bay but not at Barilla Bay included three species of goby and the galaxiid G. maculatus. Ten species were recorded in total across all sites in January, 7 in April–May, 6 in November, and 5 in July–August (Table 2). A maximum richness of four species was recorded five times. Other species observed in the marshes, but not in the pop nets are listed in Online Resource 3.
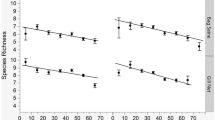
The most explanatory model for species richness consisted of water depth (67.4% concordance), location (which added 8.2% concordance), and water temperature (which added 3.8% concordance; Table 3). Mean species richness was higher at Marion Bay than at other locations (2.44 species compared to 1.5 and 0.81 at Barilla Bay and Ralphs Bay respectively). In this model, the Marion Bay sampling location and water depth had negative coefficients, while water temperature and the Ralphs Bay sampling location had positive coefficients. Species richness increased with water depth (Fig. 3). Highest species richness occurred on sampling occasions in both cold (< 12 °C) and warm (> 17 °C) water temperatures at different locations, with lower richness occurring during moderate (12–17 °C) temperatures.
Species richness per net sample by explanatory variables: a sampling location (Barilla Bay, Marion Bay, Ralphs Bay), b water depth (cm), and c water temperature (°C). Tone and shape indicate sampling location. Plot displays median line, lower and upper hinges (25th and 75th percentiles), upper and lower whiskers (extending to largest and smallest value no further than 1.5 times inter-quartile range), and outlying points
Diversity
Shannon-Weiner Index ‘H’ Diversity was best explained by water depth (68.9% concordance) and sampling location which added 3.9% concordance (Table 3). In this model, water depth had a negative coefficient while the Ralphs Bay sampling location had a positive coefficient. Diversity was highest at Marion Bay, followed by Barilla Bay, and increased with increasing water depth (Fig. 4).
Species diversity (Shannon-Weiner H′) per net sample by explanatory variables: a sampling location (Barilla Bay, Marion Bay, Ralphs Bay) and b water depth (cm). Tone and shape indicate sampling location. Plot displays median line, lower and upper hinges (25th and 75th percentiles), upper and lower whiskers (extending to largest and smallest value no further than 1.5 times inter-quartile range), and outlying points
Community Patterns
Non-metric multidimensional scaling ordination using envfit found fish community patterns were best explained by water depth (R2 = 0.149, P = 0.001) and sampling location (R2 = 0.112, P = 0.004). No other environmental parameters were found to significantly describe community patterns. Analysis of fish community and environmental relationships using BIO-ENV found water depth, water temperature, and location to be most strongly associated with fish community patterns (Fig. 5).
Fish Length
Mean A. microstoma length (TL) was only strongly influenced by month (F3,66 = 13.15, P < 0.001, R2 = 29.96%; Fig. 6). The smallest fish were recorded in January (summer; mean = 3.10 cm, standard error = 0.31). This mean differed from all others. In April–May the values were 5.38 ± 0.17 cm. Similar mean lengths were recorded in July–August (4.12 ± 0.23 cm) and November (4.73 ± 0.41 cm). The greatest range in fish lengths was recorded in November (Fig. 6).
Atherinosoma microstoma length (total length, mm) distribution in each sampling period (J/A, July/August; O, October; J, January; A/M, April/May). Plot displays median line, lower and upper hinges (25th and 75th percentiles), upper and lower whiskers (extending to largest and smallest value no further than 1.5 times inter-quartile range), and outlying points
Discussion
This study has demonstrated, for the first time, the use of temperate saltmarshes at their southern limits in Tasmania by large numbers of fish in all four seasons. Southern Tasmanian micro-tidal marshes have higher fish densities than those reported from meso-tidal marshes of northern Tasmania (Prahalad et al. 2019; Harrison-Day et al. 2022 – between 72.4 and 88 fish per 100 m2 area), and considerably higher densities than reported from mainland Australia (Prahalad et al. 2019). Notably, Marion Bay (396.9 fish per 100 m2 area) exhibited more than four times the highest previously reported densities (Prahalad et al. 2019). Even Ralphs Bay (with the lowest catch, 23 fish per 100 m2 area), has a higher density than reported from saltmarshes in mainland Australia (e.g. Thomas and Connolly 2001 – 17.2 fish per 100 m2 area, in subtropical Queensland). These results indicate that wherever fish habitat restoration is undertaken in southern temperate latitudes, it is likely to benefit fish.
Of the 17 species recorded during this study, several are common and widespread Tasmanian estuarine species. Atherinosoma microstoma, Aldrichetta forsteri, Rhombosolea tapirina, Galaxias maculatus, Pseudaphritis urvillii, Leptatherina presbyteroides, Arripis trutta, and Gymnapistes marmoratus, all recorded in the present study, are included in the ten most common species in Tasmanian upper open estuarine systems (Last 1984), the estuarine position of all saltmarshes sampled in this study. Five of these species (A. microstoma, L. presbyteroides, A. trutta, A. forsteri, and R. tapirina) are particularly common in estuarine intertidal areas (Last 1984) and are also abundant in saltmarshes in north-west Tasmania (Prahalad et al. 2019; Harrison‐Day et al. 2022). These are therefore likely to be widespread in the estuarine saltmarsh interface and can be featured as target species for fisheries management via saltmarsh restoration.
Our results confirm the important role that extent of tidal inundation plays in fish utilisation, which was seen strongly in the associations of species richness and diversity with water depth (67.4% concordance and 68.9% concordance, respectively). These findings are consistent with observations from other saltmarsh environments in which a greater number of species access the habitat during higher tides (Minello and Webb 1997; Thomas and Connolly 2001; Osgood et al. 2003). The influence of flooding depth has previously been investigated in combination with distance onto the marsh to understand overall effects of flooding characteristics (e.g. Thomas and Connolly 2001; Osgood et al. 2003; Ennis and Peterson 2015); however, the present study sought to standardise distance by sampling at similar distances from the edge. Densities of fish have been found elsewhere to be highest within 3 m of the water’s edge (Peterson and Turner 1994), so it is anticipated that the placement of sampling equipment has ensured capture of most fish using the habitat at the time of sampling. Our results suggest that restoration works should avoid reducing water depth in any way and that removing artificial barriers to inundation will increase fish abundance.
Of the other environmental variables recorded during sampling, only water temperature was found to also influence fish attributes, with pH and salinity not having any effect. Relationships between salinity and fish attributes are known to exist (e.g. Last 1984; Rakocinski et al. 1992; Ziegler et al. 2021). The lack of effect of salinity in the present study may reflect the wide tolerances of many of the species (Last 1984). Fish abundance increased with water temperature which possibly influenced fish reproduction and metabolism, thereby influencing feeding activity (Smith and Able 1994; Kneib 1997). Our results here reflect the pattern of abundance of A. microstoma which had fewest individuals approaching the coldest sampling time (Table 2).
A key finding from this study is the strong variation in fish abundance (particularly of A. microstoma but also A. forsteri) and diversity between our three sampling locations. This spatial variation may be attributable to the differences between sites in water depth and water temperature. In our study, the site with the lowest abundance and diversity (Ralphs Bay) had the lowest depths and temperatures, while the site with the highest abundance and high diversity (Marion Bay) had the highest depths and temperatures. However, in the case of Ralphs Bay, low diversity and richness extend over the full range of depths (Figs. 3 and 4), suggesting other factors may be contributing to the strong spatial variation between our sites.
An important aspect of our study design involved selecting sites that had different geomorphic settings and seascape contexts, these also being key factors in affecting fish assemblages elsewhere (Edgar et al. 1999; Saintilan et al. 2007; Baillie et al. 2015). For example, Saintilan et al. (2007) recognise the extent of shallow environments and seagrass presence and extent as major elements of seascape context in Australia. Large areas of seagrass are present close to the Marion Bay saltmarsh, but extensive seagrass loss has occurred in both Barilla Bay and Ralphs Bay (Rees 1993). While patchy seagrass beds remain at Barilla Bay, in Ralphs Bay losses of almost 100% were recorded by the 1990s (Rees 1993), with no signs of natural recovery (see Fig. 1c). This suggests that the seascape context, here the presence and extent of seagrass habitats, might suggest an alternative explanation of the spatial variation observed in this study. Previous research in these locations indicates several fish species found in this study prefer seagrass over other structured and unstructured habitats (Last 1984). Indeed, our study found species such as A. microstoma, G. marmoratus, and P. urvillli were absent or in lower numbers at Ralphs Bay, the estuary with the least seagrass. Interest in seagrass restoration in Tasmania is increasing, and future research on fish use of the saltmarsh-seagrass habitat mosaic following any future seagrass restoration project is recommended.
Furthermore, while Marion Bay and Barilla Bay (marine inlets) contain large areas of shallow water even during lowest tidal extents, Ralphs Bay (which adjoins the deep drowned river valley of the Derwent estuary) provides a much smaller area of shallow low tide habitat. It is therefore likely that these seascape habitats, and the estuarine context, influence overall fish abundance in these locations. This finding concurs with that of Edgar et al. (1999) who collected fish from Tasmanian estuaries (in shallow water, and not in saltmarsh) and explained the spatial variation in terms of salinity, seagrass biomass, tidal range, and geomorphic estuarine type. These results, as well as ours, support the conclusion of Bradley et al. (2019) that variation in seascape context can have a greater importance in determining the function of a habitat than the habitat itself.
Unlike other studies from mainland Australia that did not find a strong seasonal variation in fish found in saltmarsh (Bloomfield and Gillanders 2005; Mazumder et al. 2005), our study suggests some differences between seasons in fish abundance and length of A. microstoma. Generally, seasonal variation in fish abundance has been reported where the marsh fauna features large numbers of marine transient species with seasonal life history patterns (Rountree and Able 1992; Valiñas et al. 2012) or resident taxa with strongly seasonal breeding patterns (Talbot and Able 1984). In our study, the observed temporal differences are consistent with the breeding cycle of A. microstoma, the most abundant of saltmarsh species, an estuarine resident with a breeding period previously reported from August to January in Tasmania (Last 1984). These results suggest that multiyear monitoring programmes in these environments would be most effective if undertaken in the same season, thus controlling for likely seasonal variation in fish abundance and length. However, studies seeking to evaluate biomass should also consider seasonal differences, principally that abundances are likely to be lower after the A. microstoma breeding season.
We found that richness and diversity did not fluctuate significantly by month; however, several species occurred seasonally reflecting migratory or breeding patterns. Several of the species observed sporadically were diadromous. These included the following: G. maculatus, which has marine larvae, is an autumn–winter spawner (Last 1984; Gomon et al. 2008; Hicks et al. 2010), and was sampled as smaller individuals in August and larger in April/May; Retropinna tasmanica, which migrate from the sea and estuaries to spawn in upper estuaries and the lower reaches of coastal rivers and streams and return as larvae (Gomon et al. 2008; Hughes et al. 2014); and P. urvillii, which migrates downstream to the ocean to breed during autumn and winter (Bice et al. 2018). These observations emphasise the importance of saltmarsh as a key linking component of the landscape-to-seascape continuum.
Conclusions
Saltmarshes are a threatened ecological community globally with ongoing loss and degradation due to both direct human impacts and the effects of climate change and sea level rise. With renewed efforts to conserve and restore saltmarshes, their value and function as fish habitats are likely to provide a compelling rationale that has broad resonance among the public and policymakers. These efforts can be both informed and strengthened with locally specific data that help establish a strong baseline to quantify benefits to fisheries. The present study contributes to this knowledge base and underlines the importance of Tasmanian saltmarshes as a habitat for fish in all four seasons. Nationally high densities of fish recorded in some of these mangrove-free marshes, and the differences in fish attributes between each of the marshes provide new insights. The spatial variation in our results illustrates the need for location-specific fish use data to better inform both the selection of sites for fish habitat restoration and help model potential gains. Our findings of the potential role that seascape context reiterate that fish are likely to require other structured habitats such as seagrass when the marsh is emergent. This highlights the need for scaling up restoration of multi-habitats as part of the seascape matrix.
Data Availability
Data can be made available upon reasonable request.
References
Baillie, C.J., J.M. Fear, and F.J. Fodrie. 2015. Ecotone effects on seagrass and saltmarsh habitat use by juvenile nekton in a temperate estuary. Estuaries and Coasts 38 (5): 1414–1430.
Baker, R., B. Fry, L. Rozas, and T. Minello. 2013. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Marine Ecology Progress Series 490: 37–52.
Baltz, D.M., C. Rakocinski, and J.W. Fleeger. 1993. Microhabitat use by marsh-edge fishes in a Louisiana estuary. Environmental Biology of Fishes 36 (2): 109–126.
Bice, C.M., B.P. Zampatti, and J.R. Morrongiello. 2018. Connectivity, migration and recruitment in a catadromous fish. Marine and Freshwater Research 69 (11): 1733.
Bloomfield, A.L., and B.M. Gillanders. 2005. Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28 (1): 63–77.
Boesch, D.F., and R.E. Turner. 1984. Dependence of fishery species on salt marshes: The role of food and refuge. Estuaries 7 (4): 460.
Bradley, M., R. Baker, I. Nagelkerken, and M. Sheaves. 2019. Context is more important than habitat type in determining use by juvenile fish. Landscape Ecology 34 (2): 427–442.
Bradley, M., I. Nagelkerken, R. Baker, and M. Sheaves. 2020. Context dependence: A conceptual approach for understanding the habitat relationships of coastal marine fauna. BioScience 70 (11): 986–1004.
Bureau of Meteorology. 2021a. Tasmania in 2020: drier in the west and south, wetter in the east and warmer nights everywhere, Australian Government Bureau of Meteorology viewed 16/12/22. http://www.bom.gov.au/climate/current/annual/tas/archive/2020.summary.shtml.
Bureau of Meteorology. 2021b. Hobart – Tasmania times and heights of high and low waters, Australian Government Bureau of Meteorology, viewed 4/06/2021. http://www.bom.gov.au/oceanography/projects/ntc/tas_tide_tables.shtml.
Bureau of Meteorology. 2022. Tasmania in 2021: warmer than average statewide, typical rainfall totals for most areas, Australian Government Bureau of Meteorology, viewed 16/12/22. http://www.bom.gov.au/climate/current/annual/tas/summary.shtml#recordsRainDailyHigh.
Clarke, K.R., and R.M. Warwick. 2001. Change in marine communities: an 530 approach to statistical analysis and interpretation 2nd Edn. Plymouth Marine 531 Laboratory, Plymouth, UK.
Connolly, R.M. 2005. Modification of saltmarsh for mosquito control in Australia alters habitat use by nekton. Wetlands Ecology and Management 13 (2): 149–161.
Crinall, S.M., and J.S. Hindell. 2004. Assessing the use of saltmarsh flats by fish in a temperate Australian embayment. Estuaries 27 (4): 728–739.
Edgar, G.J., N.S. Barrett, and P.R. Last. 1999. The distribution of macroinvertebrates and fishes in Tasmanian estuaries. Journal of Biogeography 26 (6): 1169–1189.
Edgar, G.J., N.S. Barrett, D.J. Graddon, and P.R. Last. 2000. The conservation significance of estuaries: A classification of Tasmanian estuaries using ecological, physical and demographic attributes as a case study. Biological Conservation 92 (3): 383–397.
Ennis, B., and M.S. Peterson. 2015. Nekton and macro-crustacean habitat use of Mississippi micro-tidal salt marsh landscapes. Estuaries and Coasts 38 (5): 1399–1413.
França, S., M.J. Costa, and H.N. Cabral. 2009. Assessing habitat specific fish assemblages in estuaries along the Portuguese coast. Estuarine, Coastal and Shelf Science 83 (1): 1–12.
Gomon, M., D.J. Bray, and R.H. Kuiter. 2008. Fishes of Australia’s southern coast, Reed New Holland, Chatswood, N.S.W.
Harrison-Day, V., V. Prahalad, J.B. Kirkpatrick, and M. McHenry. 2020. A systematic review of methods used to study fish in saltmarsh flats. Marine and Freshwater Research 72 (2): 149–162.
Harrison‐Day, V., V. Prahalad, M.T. McHenry, J. Aalders, and J.B. Kirkpatrick. 2022. Introduced Spartina anglica modifies fish habitat in southern temperate succulent saltmarshes. Restoration Ecology 31 (7): 1–13.
Hettler, W. 1989. ’Nekton use of regularly-flooded salt-marsh cordgrass habitat in North Carolina. USA’, Marine Ecology Progress Series 56: 111–118.
Hicks, A., N.C. Barbee, S.E. Swearer, and B.J. Downes. 2010. Estuarine geomorphology and low salinity requirement for fertilisation influence spawning site location in the diadromous fish, Galaxias maculatus. Marine and Freshwater Research 61 (11): 1252.
Hughes, J.M., D.J. Schmidt, J.I. Macdonald, J.A. Huey, and D.A. Crook. 2014. Low interbasin connectivity in a facultatively diadromous fish: Evidence from genetics and otolith chemistry. Molecular Ecology 23 (5): 1000–1013.
Inc, Minitab. 2010. Minitab users guide release 16. USA: Minitab Inc.
Inland Fisheries Act 1995 (Tasmania). https://www.legislation.tas.gov.au/view/html/inforce/current/act-1995-110.
Kelleway, J.J., K. Cavanaugh, K. Rogers, I.C. Feller, E. Ens, C. Doughty, and N. Saintilan. 2017. Review of the ecosystem service implications of mangrove encroachment into salt marshes. Global Change Biology 23 (10): 3967–3983.
Kneib, R.T. 1987. Predation risk and use of intertidal habitats by young fishes and shrimp. Ecology 68 (2): 379–386.
Kneib, R. 1991. Flume weir for quantitative collection of nekton from vegetated intertidal habitats. Marine Ecology Progress Series 75: 29–38.
Kneib, R.T. 1997. Early life stages of resident nekton in intertidal marshes. Estuaries 20 (1): 214.
Kumbier, K., M.G. Hughes, K. Rogers, and C.D. Woodroffe. 2021. Inundation characteristics of mangrove and saltmarsh inmicro-tidal estuaries. Estuarine, Coastal and Shelf Science 261: 1–13.
Land Tasmania. 2015. LIST Ramsar Wetlands, Land Tasmania, 2015, http://maps.thelist.tas.gov.au/listmap/app/list/map?layout-options=LAYER_LIST_OPEN&cpoint=147.43,-42.85,50000&srs=EPSG:4283&bmlayer=3&layers=161.
Last, P. 1984. Aspects of the ecology and zoogeography of fishes from soft-bottom habitats of the Tasmanian shore zone. PhD thesis, University of Tasmania.
Living Marine Resources Management Act 1995 (Tasmania). https://www.legislation.tas.gov.au/view/html/inforce/2024-03-18/act-1995-025.
Lucieer, V.L. 2007. SeaMapTasmania Habitat Data, Tasmanian Aquaculture and Fisheries Institute, http://metadata.imas.utas.edu.au/geonetwork/srv/eng/metadata.show?uuid=7a6751e0-1ad5-11dc-9e36-00188b4c0af8.
Mariano-Jelicich, R., G. García, and M. Favero. 2014. Fish composition and prey utilization of the black skimmer (Rynchops niger) in mar Chiquita coastal lagoon, Argentina. Brazilian Journal of Oceanography 62 (1): 1–10.
Mazumder, D., N. Saintilan, and R.J. Williams. 2005. Temporal variations in fish catch using pop nets in mangrove and saltmarsh flats at Towra Point, NSW, Australia. Wetlands Ecology and Management 13 (4): 457–467.
Mazumder, D., N. Saintilan, and R. Williams. 2006a. Trophic relationships between itinerant fish and crab larvae in a temperate Australian saltmarsh. Marine and Freshwater Research 57 (2): 193–199.
Mazumder, D., N. Saintilan, and R.J. Williams. 2006b. Fish assemblages in three tidal saltmarsh and mangrove flats in temperate NSW, Australia: A comparison based on species diversity and abundance. Wetlands Ecology and Management 14 (3): 201–209.
Mazumder, D., N. Saintilan, R. Williams, and R. Szymczak. 2011. Trophic importance of a temperate intertidal wetland to resident and itinerant taxa: Evidence from multiple stable isotope analyses. Marine and Freshwater Research 62: 11.
McPhee, J.J., M.E. Plate, and M.J. Schreider. 2015. Trophic relay and prey switching - a stomach contents and calorimetric investigation of an ambassid fish and their saltmarsh prey Estuarine. Coastal and Shelf Science 167: 67–74.
Minello, T., and J. Webb. 1997. ’Use of natural and created Spartina alterniflora salt marshes by fishery species and other aquatic fauna in Galveston Bay. Texas, USA’, Marine Ecology Progress Series 151: 165–179.
Odum, E.P. 2002. Tidal marshes as outwelling/pulsing systems. In Concepts and Controversies in Tidal Marsh Ecology, ed. M.P. Weinstein and D.A. Kreeger, 3–7. New York: Kluwer Academic Publishers.
Oksanen, J., G. Blanchet, R. Kindt, P.R. Minchin, P. Legendre, B. O’hara, G.L. Simpson, P. Solymos, M.H.H. Stevens, and H. Wagner. 2011. vegan: community ecology package. R package version 2.0–2.0 R Foundation for Statistical Computing, Vienna, Austria.
Osgood, D.T., D.J. Yozzo, R.M. Chambers, D. Jacobson, T. Hoffman, and J. Wnek. 2003. Tidal hydrology and habitat utilization by resident nekton in Phragmites and non-Phragmites marshes. Estuaries 26 (2): 522–533.
Palmer, K., C. Watson, and A. Fischer. 2019. ’Non-linear interactions between sea-level rise, tides, and geomorphic change in the Tamar Estuary. Australia’, Estuarine, Coastal and Shelf Science 225: 106247.
Peterson, G.W., and R.E. Turner. 1994. The value of salt marsh edge vs interior as a habitat for fish and decapod crustaceans in a Louisiana tidal marsh. Estuaries 17 (1): 235.
Platell, M.E., and P. Freewater. 2009. Importance of saltmarsh to fish species of a large south-eastern Australian estuary during a spring tide cycle. Marine and Freshwater Research 60 (9): 936–941.
Prahalad, V., and J.B. Kirkpatrick. 2019. Saltmarsh conservation through inventory, biogeographic analysis and predictions of change: Case of Tasmania, south-eastern Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 29 (5): 717–731.
Prahalad, V., V. Harrison-Day, P. McQuillan, and C. Creighton. 2019. Expanding fish productivity in Tasmanian saltmarsh wetlands through tidal reconnection and habitat repair. Marine and Freshwater Research 70 (1): 140.
Quinn, G.P., and M.J. Keough. 2002. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press.
R Core Team. 2019. A language and environment for statistical computing In: Computing RFFS (ed) Vienna, Austria.
Rakocinski, C.F., D.M. Baltz, and J.W. Fleeger. 1992. Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary. Marine Ecology Progress Series 80 (2–3): 135–148.
Rees, C.G. 1993. Tasmanian seagrass communities. Masters thesis, University of Tasmania.
Rountree, R.A., and K.W. Able. 2007. Spatial and temporal habitat use patterns for salt marsh nekton: Implications for ecological functions. Aquatic Ecology 41 (1): 25–45.
Rountree, R.A., and K.W. Able. 1992. Fauna of polyhaline subtidal marsh creeks in Southern New Jersey: composition, abundance and biomass. Estuaries 15 (2): 171.
Rozas, L.P. 1995. Hydroperiod and its influence on nekton use of the salt marsh: A pulsing ecosystem. Estuaries 18 (4): 579.
Rstudio Team. 2016. RStudio: integrated development for R. In: Rstudio I. (ed). Boston MA USA.
Saintilan, N., K. Hossain, and D. Mazumder. 2007. Linkages between seagrass, mangrove and saltmarsh as fish habitat in the Botany Bay estuary, New South Wales. Wetlands Ecology and Management 15 (4): 277–286.
Smith, K.J., and K.W. Able. 1994. Salt-marsh tide pools as winter refuges for the mummichog, Fundulus heteroclitus, in New Jersey. Estuaries 17 (1): 226.
Swamy, V., P.E. Fell, M. Body, M.B. Keaney, M.K. Nyaku, E.C. McIlvain, and A.L. Keen. 2002. Macroinvertebrate and fish populations in a restored impounded salt marsh 21 years after the reestablishment of tidal flooding. Environmental Management 29 (4): 516–530.
Talbot, C.W., and K.W. Able. 1984. Composition and distribution of larval fishes in New Jersey high marshes. Estuaries 7 (4): 434.
Thomas, B.E., and R.M. Connolly. 2001. Fish use of subtropical saltmarshes in Queensland, Australia: Relationships with vegetation, water depth and distance onto the marsh. Marine Ecology Progress Series 209: 275–288.
Valiñas, M.S., L.M. Molina, M. Addino, D.I. Montemayor, E.M. Acha, and O.O. Iribarne. 2012. Biotic and environmental factors affect Southwest Atlantic saltmarsh use by juvenile fishes. Journal of Sea Research 68: 49–56.
Visby, I., and V. Prahalad. 2020. Planning for the future: Derwent Estuary saltmarsh baseline monitoring and management, Derwent Estuary Program, Hobart. https://www.derwentestuary.org.au/assets/Visby_Prahalad_July2020__Derwent_Estuary_Saltmarsh_Report.pdf.
Warren, R.S., P.E. Fell, J.L. Grimsby, E.L. Buck, G.C. Rilling, and R.A. Fertik. 2001. Rates, Patterns, and Impacts of Phragmites australis expansion and effects of experimental phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the Lower Connecticut River. Estuaries 24 (1): 90.
Warren, R.S., P.E. Fell, R. Rozsa, A.H. Brawley, A.C. Orsted, E.T. Olson, V. Swamy, and W.A. Niering. 2002. Salt marsh restoration in Connecticut: 20 years of science and management. Restoration Ecology 10 (3): 497–513.
West, J.M., and J.B. Zedler. 2000. Marsh-creek connectivity: Fish use of a tidal salt marsh in Southern California. Estuaries 23 (5): 699.
Ziegler, S.L., R. Baker, S.C. Crosby, D.D. Colombano, M.A. Barbeau, J. Cebrian, R.M. Connolly, L.A. Deegan, B.L. Gilby, D. Mallick, C.W. Martin, J.A. Nelson, J.F. Reinhardt, C.A. Simenstad, N.J. Waltham, T.A. Worthington and L.P. Rozas. 2021. Geographic variation in salt marsh structure and function for nekton: a guide to finding commonality across multiple scales. Estuaries and Coasts 44: 1497–1507.
Acknowledgements
We thank the many field volunteers who assisted with data collection for this project, and John Pogonoski (CSIRO, Australian National Fish Collection) for assistance with fish identification. This research was carried out following University of Tasmania Animal Ethics permit conditions (A0018547) and with approval from Tasmanian DPIPWE Water and Marine Resources Division (Living Marine Resources Management Act 1995 Research Sampling Permit, Permit No. 20151 and Permit No. 19190), Inland Fisheries Service (Inland Fisheries Act 1995 Exemption Permit, Permit No. 2020-20), and Marine Conservation Program, Wildlife Management Branch (Permit to Take Wildlife for Scientific Purposes, Authority No. FA 20161). Thanks to Clarence City Council, Tasmanian Parks and Wildlife Services Property Services, and Tony Byrne for providing permission to carry out research at Ralphs Bay, Marion Bay, and Barilla Bay.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was supported by an Australian Government Research Training Program (RTP) Scholarship and by Holsworth Wildlife Research Endowment funding.
Author information
Authors and Affiliations
Contributions
VHD, VP, JBK, MTM, and JA conceived and designed the research. VHD, JA, and VP collected the data. VHD, VP, and JBK, acquired funding and permits. VHD, JBK, MTM, and VP analysed and interpreted the data. VHD wrote the initial draft. All authors edited the manuscript. JBK, VHD,VP revised the manuscript. Their revisions were edited by the other authors.
Corresponding author
Additional information
Communicated by Nathan Waltham
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harrison-Day, V., Kirkpatrick, J.B., Prahalad, V. et al. The Effect of Location, Time, and Environmental Conditions on Fish Use of Southern Temperate Saltmarshes. Estuaries and Coasts 47, 1086–1100 (2024). https://doi.org/10.1007/s12237-024-01340-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01340-z