Abstract
The impact of urban and agricultural development on sediment quality in the Alvarado Lagoon region in the southern Gulf of Mexico requires an examination of the historical behavior of potential toxic metals (PTMs) and polycyclic aromatic hydrocarbons (PAHs). Consequently, this study aims to assess the ecotoxicological hazards that benthic species and human consumers face in the area. These results are crucial for economic activities in the region and can help prevent future hazards. We examined two sediment profiles from the ecosystem: Profile 1, which spans the period between 1929 and 1998, and Profile 2, which covers the years between 1929 and 2007. The study evaluated the degree of human-induced pollution of six trace metal elements (PTMs): arsenic (As), cadmium (Cd), chromium (Cr), nickel (Ni), lead (Pb), and vanadium (V) in the sediments of Alvarado Lagoon. The Enrichment Factor (EF) and Geoaccumulation Index (IGeo) were computed as internationally recognized indices to measure the magnitude of contamination and additional anthropogenic and geochemical inputs contributing to the natural levels of the elements. Our analysis indicates that there is no evidence of either enrichment or pollution (EF < 1 class 1; IGeo < 0 class zero) found in the sediments of Alvarado Lagoon. The occurrence of these elements can be attributed to their lithogenic origin, as supported by a significant correlation observed between them. Within the 16 polycyclic aromatic hydrocarbons (PAHs) analyzed, solely Naphthalene (Nap) and Phenanthrene (Phe) were identified in both sediment profiles. The levels of chemicals are indicative of minimal ecotoxicological risks, with Nap ranging between 0.25 and 0.43 µg g−1 and Phe ranging between 0.31 and 0.79 µg g−1. The analysis of factors in this study identified two distinct factors, one related to lithogenic processes and another related to petrogenic processes. The sedimentary profiles of the study site confirmed low levels of potentially toxic metals and polycyclic aromatic hydrocarbons (PAHs), posing insignificant environmental risks. As a result, the ecosystem in this area has demonstrated resilience.
Similar content being viewed by others
Introduction
The global rise in population and urban development has led to an increase in agricultural, livestock, industrial, and commercial activities, which has caused the degradation of coastal ecosystems (Estevez et al. 2016; Ontiveros-Cuadras et al. 2019; Irabien et al. 2019; Botello et al. 2022; Ruiz-Fernández et al. 2022; Lacoste et al. 2023; Rafiei et al. 2023). These inputs come from various sources, including wastewater, industrial and domestic discharges, as well as runoff (Zhao et al. 2017; Algül and Beyhan 2020; Liu et al. 2020; Xiao et al. 2021; Shi et al. 2022).
The most substantial environmental harm arises when the discharge of polluting waste from these activities exceeds the capacity of ecosystems to assimilate or degrade them or when organisms cannot detoxify them. Sediments are the final destination of most pollutants that reach coastal areas. The removal and resuspension caused by fishing and recreational activities (such as the transit of boats and pangas), can elevate pollutants in the water column; this increase in toxicity can harm nektonic organisms, ultimately affecting humans through the trophic web. Within the anthropogenic pollutants in the aquatic environment, there are two significant groups: Potentially Toxic Metals (PTMs) and Polycyclic Aromatic Hydrocarbons (PAHs) (Ruiz-Fernández et al. 2012; Ponce-Vélez and de la Lanza-Espino 2019; Valentina et al. 2021). High contents of both pollutants are adsorbed in aquatic sediments through complex chemical and physical mechanisms. High levels of PTMs and PAHs can have adverse effects on benthic organisms, decreasing their biodiversity and abundance. Due to their persistence, bioaccumulation capacity, and toxicity across food webs, both PTMs and PAHs have the potential to threaten ecosystems and human health (Mountouris et al. 2002; Dalia et al. 2014; Sutilli et al. 2020; Neves et al. 2022; Di Duca et al. 2023).
Through various physicochemical mechanisms (including adsorption, coagulation, precipitation, and ion exchange) that occur at the water–sediment interface (Qasem et al. 2021; Yuan et al. 2022), it is possible for metals (dissolved or as insoluble chemical species) to be deposited in sediments which can then serve as suppliers of the same metals based on changes in the physicochemical conditions prevailing on the aquatic ecosystem bed (Milligan and Law 2013; Seelen et al. 2018; Bastakoti et al. 2019; Armstrong-Altrin et al. 2019; Li et al. 2023).
On the contrary, PAHs are persistent compounds that are comprised of two or more benzene rings, with low solubility in water, and are linked via intricate chemical and physical adsorption mechanisms to the humic fraction of detritus. They are present throughout in aquatic environments as pollutants, particularly in regions with human influence like ports, estuaries, and other coastal zones (Cornelissen et al. 2006). PAHs have been detected in the marine environment following oil spills since 1967 (Balcioglu 2016). These compounds were also reported to be present in food and cigarette smoke and were subsequently detected in air samples due to motor vehicle exhaust (Nguyen et al. 2014; Balcioglu 2016). These properties also enable them to distribute widely in the biosphere, accumulate in various food webs, and be transported worldwide (Yunker et al. 2015). The physicochemical characteristics of PAHs vary based on their molecular weight, as the molecular weight increases, the aqueous solubility decreases, and the melting point, boiling point and log KOW (octanol/water partition coefficient) increase, indicating greater solubility in lipids that can lead to their bioaccumulation in the adipose tissues of organisms and cause harmful effects on their development, reproduction and immunological functions (Balcioglu 2016). They come from both natural and anthropogenic sources (WHO 2003). They can be produced by organisms (biogenic), or derived from rapid incineration processes at high temperatures (pyrolytic), or from fossil fuels or slow burning of organic matter (petrogenic), or originate from transformation processes in soils and sediments (diagenetic) (Hylland 2006; Balcioglu 2016). During the 1950s, due to their carcinogenic and mutagenic effects of these pollutants on human health, their distribution in various environmental compartments has been extensively studied (Nguyen et al. 2014; Suman et al. 2016; Balgobin and Ramroop 2019; Mihankhah et al. 2020; Wang et al. 2020; Montuori et al. 2022).
Because anthropogenic contributions from agricultural, livestock, urban and industrial activities are related to high levels of these contaminants in coastal aquatic systems, it is important to document the accumulation of this xenobiotic mixture in environments such as the Alvarado Lagoon in the south of the Gulf of Mexico, of economic importance due to the fishing activities that have been carried out for decades. This lagoon receives discharges from the Blanco and Papaloapan rivers that cross livestock, agricultural and industrial areas, transporting terrigenous materials contaminated with pesticides, heavy metals and PAHs from waste from agricultural, livestock and industrial practices, from logging and burning of soils, agriculture and forest fires in the dry season (Mallén et al. 2006; Nava-Tablada and Villanueva-Fortanelli 2021; Villas-Hernández et al. 2022). The Alvarado Lagoon has become a container for toxic substances that have accumulated in the sediments and are available to be incorporated by the benthos (Guentzel et al. 2011; Ruiz-Fernández et al. 2014; Botello et al. 2018; Ponce-Vélez and de la Lanza-Espino 2019; Velandia-Aquino et al. 2023). In this manner, analyzing lagoon sediment profiles along with the corresponding dating enables us to understand the historical trend of these hazardous pollutants and identify usage patterns, the impact of weather events, human activities, and other natural and/or human-induced processes. The information allows us to predict future environmental behaviors and provide valuable data for effective decision-making regarding the management of these ecosystems and its resources. Furthermore, it promotes sustainable activities in the areas surrounding these coastal sites (Ruiz-Fernández and Páez-Osuna 2011; Armstrong-Altrin and Pineda-Natalhy 2014; Armstrong-Altrin et al. 2019; Botello et al. 2022; Ruiz-Fernández et al. 2022; Velandia-Aquino et al. 2023).
Given the various factors that have affected the ecosystem under study, the following research hypothesis was proposed: in the sediments of the Alvarado Lagoon, there is a record of the contaminants that have gradually been deposited in it, and it is considered that there is an origin common anthropogenic among them. The study had as objectives the following: (1) evaluate the historical behavior of Potentially Toxic Metals (PTMs) and Polycyclic Aromatic Hydrocarbons (PAHs) in two sedimentary profiles recovered from the Alvarado Lagoon; (2) quantify the degree of contamination and the impact of anthropogenic geochemistry and (3) establish any possible correlation between these contaminants and estimate the potential environmental risk.
Materials and Methods
Area of Study
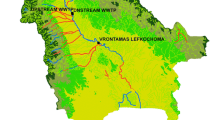
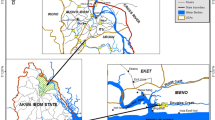
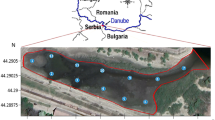
The Alvarado Lagoon System, which is in the central portion of the state of Veracruz in the southern Gulf of Mexico, includes the Alvarado Lagoon which measures about 2212.5 ha and is the largest among the four main lagoons: the others namely, Camaronera, Buen País, and Tlalixcoyan. It receives runoff of the Papaloapan, Blanco, and Acula Rivers, and communicates with the sea through its Inlet with an amplitude of 400 m (Fig. 1). The study area has three climatic seasons: July–October rainfall, October–February “northerns”, and March–June dry season. The entire perimeter of the wetland is encompassed by mangrove forests, along with halophyte grasses, palm trees, and other trees (CONABIO 1998; Vázquez et al. 2009). Additionally, it is the third more extensive wetland in Mexico, it has been declared a RAMSAR site as it serves as a haven for various birds, fishes and crustaceans populations for feeding and reproduction (Guentzel et al. 2011).
Sampling
In May 2021, two sedimentary profiles were collected in the Alvarado Lagoon, P1 (18°4 8′15.73″N, 95°4 9′35.15″W) and P2 (18°47′5.22″N, 95°47′9.512″W). P1 was located in an area affected by the Papaloapan River’s fluvial influence, while P2 was subjected to fluvial influences from the Papaloapan, Blanco, and Acula Rivers as well as marine influence from the Gulf of Mexico. Additionally, both profiles receive some anthropogenic influence from Alvarado Port and the nearby agricultural, livestock, industrial and fishing areas. The sampling areas were selected according to the Guidelines outlined in the Regional Project Manual RLA/7/012/ (IAEA 2009). Transparent acrylic tubes (10 cm inner diameter) were used to extract the profiles. They were transported in a container covered with ice and kept in upright position a temperature of 5 °C in the laboratory until further processing. The P1 profile was 24 cm long while the P2 profile was 50 cm; both were divided into strata of varying thicknesses: P1 was divided every 6 cm (3, 9, 15, and 21 cm) and P2 every 5 cm (2.5, 7.5, 12.5, 17.5, 22.5, 27.5, 32.5, 37.5, 42.5, and 47.5 cm). For P1, the age of the sediments was estimated at 69 years (1929 to 1998) which corresponds to the previously dated core in Botello et al. (2018), while for P2 the age of 78 years (1929 to 2007) which corresponds to the core (N3) previously dated in Velandia-Aquino et al. (2023). Each sedimentary column was inverted and extruded using a plunger and cut transversely, depositing each stratum on a glass plate. The sediments were dried in the open air at an ambient temperature ranging between 26 and 29 °C, then homogenized in a porcelain mortar and sieved through a 250 µm mesh and stored in plastic bags for analysis of Potentially Toxic Metals (PTMs) and in glass jars for analysis for Polycyclic Aromatic Hydrocarbons (PAHs).
To determine the historical fluxes of PTMs and PAHs in the two profiles (P1 and P2), two sedimentary cores recovered from the Alvarado Lagoon System were used as reference, which were dated with the isotopes 210Pb and 137Cs (Botello et al. 2018; Velandia-Aquino et al. 2023).
Potentially Toxic Metals (PTMs) Analysis
The PTMs analysis was conducted using the US EPA 6010D-2018 method (INTERTEK + ABC Analitic). To digest samples, Method 3052 was employed. The dry sediments, ground and sieved, were weighed 0.5 ± 0.1 g per stratum, and digestion was done in the Anton Paar Multiwave 3000 microwave equipment, using 12 mL of nitric acid (HNO3) and 4 mL of hydrochloric acid (HCl) per sample and centrifuged for 5 min at 1500 rpm. The elements arsenic (As), cadmium (Cd), chromium (Cr), nickel (Ni), vanadium (V), lead (Pb), and aluminum (Al) were identified and quantified using the inductively coupled plasma optical emission spectrophotometer (ICP-OES) model Thermo Scientific, brand ICAP 7400. Internal certified standards were used for quality control. The relative standard deviations of the blanks and replicates were below 5%, and the recoveries (90–100%) of the standard reference materials were: 98.7% Al; 93.7% As; 94.5% Cd; 98.6% Cr; 97.7% Ni; 98.7 Pb and 97.4% V, and the coefficients of variation were: 2.7% Al; 2.6% As; 1.7% Cd; 1.4% Cr; 0.5% and Ni; 1.5% Pb and 2.0% V. The detection limits for each metal were: Cd, Cr, and V 0.001 μg·g−1; Al 0.002 μg·g−1; As 0.005 μg·g−1; and Pb 0.007 μg·g−1. This laboratory is accredited by the EMA (Mexican Accreditation Entity) Testing Laboratory and has the legal recognition of COFEPRIS (Federal Commission for the Protection against Sanitary Risks), CONAGUA (National Water Commission), and PROFEPA (Federal Attorney for Environmental Protection) among others. It has obtained the Certificate of Conformity of Quality Assessments ABS ISO 14001:2004 and has evolved to ISO 14001:2015.
Enrichment Factor (EF)
The EF evaluates the degree of enrichment or contamination in sediments by the presence of metals. It compares the current concentration of metal in the ecosystem with the average Earth’s crust values and normalized with reference elements such as Al, Ti or Fe, which are uniformly sourced in the upper continental crust. The EF (Förstner and Wittmann 1979) is calculated with the equation \(EF=\frac{\left[\nicefrac{X}{B}\right]sediment}{\left[ \nicefrac{X}{B}\right]crust}\) where \({X}_{sediment}\) and \({X}_{crust }\) are the concentration of the metal (X) in the sample and in local geological background materials respectively; \({B}_{sediment}\) and \({B}_{crust}\) are the concentrations of a conservative element chosen to normalize the data in the sample and in geological background materials. Seven classes of enrichment based on EF are recognized: class 1 (EF < 1) there is no enrichment; class 2 (1 ≤ EF < 3) minor enrichment; Class 3 (3 ≤ EF < 5) moderate enrichment; class 4 (5 ≤ EF < 10); moderately severe enrichment; class 5 (10 ≤ EF < 25) severe enrichment; class 6 (25 ≤ EF < 50) very severe enrichment; class 7 (EF ≥ 50) extremely severe enrichment. To evaluate the anthropogenic contribution of metals in the Alvarado Lagoon, the EF was calculated using aluminum as a conservative element to normalize the data. This choice is justified as Al predominantly originates from aluminosilicates derivate from the weathering of the continental crust. The metal concentrations previously reported for the upper continental crust were used as the geological background (Rudnick and Gao 2003).
Geoaccumulation Index (IGeo)
The IGeo (Müller 1979) assesses the degree of metal contamination comparing current concentration levels with pre-industrial levels. The IGeo is calculated with the equation \({I}_{Geo}={\mathit{log}}_{2}\left[^{{C}_{sediment}}/ _{{1.5C}_{crust}}\right]\) (Ridgway and Shimmeld 2002; Rajan et al. 2008) where \({C}_{sediment}\) is the concentration (μg·g−1) of the metal examined and \({C}_{crust}\) is the background geochemical concentration of the same metal (μg·g−1); the value of 1.5 is a factor that considers possible variability generated by lithological variations. The IGeo is composed of seven grades, IGeo ≤ 0 class 0 uncontaminated; 0 < IGeo ≤ 1 class 1 from uncontaminated to moderately contaminated; 1 < IGeo ≤ 2 class 2 moderately contaminated; 2 < IGeo ≤ 3 class 3 from moderate to heavily contaminated; 3 < IGeo ≤ 4 class 4 heavily contaminated; 4 < IGeo ≤ 5 class 5 from strong to extremely contaminated; IGeo > 5 class 6 extremely contaminated (Praavena et al. 2008). In this study, Upper Continental Crust values were used as background or pre-industrial levels (Rudnick and Gao 2003).
Polycyclic Aromatic Hydrocarbons (PAHs) Analysis
The 16 polycyclic aromatic hydrocarbons (PAHs) designated as USEPA priority toxic pollutants (USEPA 2009) were analyzed: Naphthalene (Nap), Acenaphthylene (Ac), Acenaphthene (Ace), Fluorene (Fl), Phenanthrene (Phe), Anthracene (An), Fluoranthene (Flu), Pyrene (Py), Benzo[a] anthracene (BaA), Chrysene (Cr), Benzo[b]fluoranthene (BbF), Benzo[k]fluoranthene (BkF), Benzo[a]pyrene (BaP), Dibenzo[a,h]anthracene (DBA), Indeno[1,2,3-cd]pyrene (IP), and Benzo[g,h,i]perylene (BghiP). The analysis was conducted in the Marine Pollution Laboratory, in adherence to technical guidelines outlined in Methods EPA 3546 and 3620. The instrumental analysis was carried out on an Agilent Gases (6890) – Masses (5973N) equipment with the assistance of the Laboratory of Chemical Speciation of Atmospheric Organic Aerosols of the Institute of Atmospheric Sciences and Climate Change of the UNAM. The detection limit was 0.1 pg·g−1 dry weight.
Sedimentary Quality Index
To assess the health of the ecosystem, the sedimentary quality criteria SQuiRTs (Screening Quick Reference Table for Inorganics and Organics in Sediment) by the National Oceanic and Atmospheric Administration (NOAA) (Buchman 2008) were used as a tool to interpret chemical data from the sedimentary environment and associate them with possible adverse biological effects on the biota. The concentrations of PTMs obtained were compared with the values of the ERL (Effects Range Low) and ERM (Effects Range Median) of the SQuiRTs for the metals (µg·g−1): ERL (As 8.2; Cd 1.2; Cr 81; Pb 46.7 y Ni 20.9) and ERM (As 70; Cd 9.6; Cr 370; Pb 218 y Ni 51.6). Similarly, the concentrations of the 16 PAHs were compared with the values (ng·g−1) of ERL and ERM: Acenaphthylene (44, 640), Acenaphthene (16, 500), Anthracene (85.3, 1100), Benzo[a]anthracene (261, 1600), Benzo[b]fluoranthene (not applicable), Benzo[k]fluoranthene (not applicable), Benzo[a]pyrene (430, 1600), Benzo[ghi]perylene (not applicable), Chrysene (384, 2800), Dibenz[ah]anthracene (63.4, 260), Fluoranthene (600, 1500), Fluorene (19, 540), Indeno[1,2,3-cd]pyrene (not applicable), Naphthalene (Na)(160, 2100), Phenanthrene (240, 1500), Pyrene (665, 2600) and ΣPAHs (4022, 44,792).
Statistical Analysis
Several statistical tests were applied to explain PTMs and PAHs behavior in the sediments of Alvarado Lagoon using the computer program Statistica version 8 and Minitab 19. Initially, the normal distribution of the data was determined by using the Kolmogorov–Smirnov statistical test. Subsequently, Pearson’s correlation statistical test was applied to measure the linear relationship between the variables and these results were contrasted with the Cluster test and dendrogram plot. Next, multivariate analyses using Factor Analysis and Principal Components Analysis (PCA) were performed, which are methods for extracting variability within a set of possibly correlated variables.
Results
Two sedimentary profiles (P1 and P2) were recovered in the Alvarado Lagoon, both sequences were compact and homogeneous with no signs of bioturbation, lamination, or mixing. To determine the age of the sediments of each profile, they were correlated with the previously dated cores and the strata were sectioned to match the reference cores.
Content of Potentially Toxic Metals (PTMs)
Concentration ranges (μg·g−1) of the PTMs (Table 1) were for P1: Cr 23.9–30.3; Ni 13.8–16.1; Pb 7.2–10.1; V 24.8–35.1, and for Al 15.1 mg⋅g−1–21.7 mg⋅g−1, while As and Cd were not detected (< 1.0 μg·g−1). For P2, as in P1, As and Cd were not detected and for the rest of the PTMs, the concentration intervals (μg·g−1) were Cr 16.3–25.3; Ni 10.5–14.8; Pb 5.3–10.3; V 17.5–29.4, and Al 15.1 mg⋅g−1–21.7 mg⋅g−1 (Table 1).
The concentrations of PTMs in P1 were from highest to lowest concentration Al > V > Cr > Ni > Pb > Cd > As. The deposition trends were similar for Ni, Cr, and V, with an increase in the oldest sediments until the 1970s and a subsequent decrease towards more recent sediments. In contrast, the Pb exhibited an increase in the oldest sediments peaked in the late 1940s, decreased until the end of the 1960s, and remaining stable since the 1970s (Fig. 2a). In P2, the concentrations of PTMs were from highest to lowest concentration Al > V > Cr > Ni > Pb > Cd > As. The deposition trends were similar for all the analyzed elements following a similar pattern, with periodic oscillations, indicating eight turning points. There were four points of maximum concentrations in the years 1957, 1971, 1989, and 2007 and four of minimum concentrations in 1950, 1964, 1980, and 1998 (Fig. 2b). Pb is the only metal that exhibits a consistent behavior since the mid-seventies, coinciding with the gradual decrease of Pb in gasoline, by eliminating the tetraethyl lead that was used in gasoline to increase octane. In this way, atmospheric emissions of Pb from automotive traffic were reduced, since previously it was considered that the most important source of Pb in coastal ecosystems was fuel residues released into the atmosphere by floating and rolling traffic, as well as wastewater discharged into the ecosystem (Hung and Hsu 2004).
The enrichment factor for As, Cd, Cr, Ni, Pb, and V had values < 1 (class 1) indicating that there is no enrichment of these metals in the sediments of both sedimentary profiles (Table 2). The IGeo calculated for all PTMs were < 0 (class zero) classifying both profiles as uncontaminated sediments. The low concentrations of PTMs in both profiles and the resulting calculations of the EF and Igeo suggest that the anthropogenic activities in the surrounding study area have a minimal impact.
Content of Polycyclic Aromatic Hydrocarbons (PAHs)
In the process of identifying and quantifying the 16 PAHs of interest, only two compounds were found to be present in both profiles (Table 1), accounting 12.5% of the total hydrocarbons sought regarding the ΣPAH in both profiles. The concentrations of both compounds in P1 exhibited similar deposition trends, albeit with varying maximum concentrations; Phe achieved its highest concentration around 1950 (0.58 pg·g−1), whereas Nap recorded the highest value in 2005 (0.33 pg·g−1) (Fig. 3a). For P2 both compounds exhibited similar behavior over time. In 1964, where Phe had a higher concentration of 0.79 pg·g−1, but it decreased by almost half (0.33 pg·g−1) in the year 2007; Nap, on the other hand, reached its maximum concentration peak of 0.43 pg·g−1, in 1988 and maintained concentrations from 0.25 to 0.43 pg·g−1 throughout the entire profile as shown in Fig. 3b.
Statistical Analysis
Table 3 displays the Pearson correlation matrix for PTMs and PAHs. The results indicate high positive correlation (p < 0.05) between the metals Cr-Al (0.95), V-Cr (0.93), Ni–Cr (0.84), V-Al (0.83) and V-Ni (0.81). Additionally, Nap displayed a moderate positive correlation with Phe (0.47), and a moderate negative correlation with the metals V (− 0.54), Cr and Ni (− 0.47), Al (− 0.44), and Pb (− 0.42).
In the Cluster test (Fig. 4) conducted on the analyzed parameters, the results of the amalgamation showed a slight decrease in the level of similarity from step 1 (97.5) to step 2 (91.3). The similarity then decreases moderately at step 3 (85.8), and thereafter, a sharp decrease was observed at step 4 (76.4%) and step 5 (73.7%). These findings suggest that 5 clusters are appropriate for the final partition. The dendrogram (Fig. 4) was sliced at a similarity of around 73.7% leaving resulting in four final conglomerates. The first linked Al-Cr, and V. The second conglomerate linked Ni, and the third conglomerate contains Pb. Additionally, a coalition between Nap-Phe was observed in the fourth conglomerate.
Table 1-S displays the cumulative ratio used to determine the total amount of variance explained by the Principal Components and meeting Kayser’s criterion. Sedimentation plot (Fig. 5a) identified 2 components (4.354, 1.335) that account for most the data variation (81.3%). The Principal Components plot (Fig. 5b) displays results for the first two components. Component 1 (F1) explains 62.2% of total variance and reveals large positive influences of Cr, V and Al. Component 2 (F2), shows Phe to have a high positive influence (0.94), followed by a moderately high Nap (0.54) and explains 19.1% of the total variance (Table 2-S).
Discussion
Content of Potentially Toxic Metals (PTMs)
The two sediment profiles exhibit fluctuations in PTM concentrations. The maximum concentrations in both profiles may be linked to meteorological occurrences in the Mexican region during the dates in which these peaks were measured; the high levels of precipitation in these systems result in a greater sediment load from the high areas of the Papaloapan Basin towards the coastal plain of Veracruz where the Alvarado Lagoon System is located. The Alvarado Lagoon is the largest in the area and directly receives the discharges of the Papaloapan, Blanco and Acula Rivers, as well as influences from the Gulf of Mexico through the entrance of the lagoon. The turning points of maximum concentration (1957, 1971, 1989 and 2007) identified in P2 could be due to the accumulation of sediments caused by meteorological events (García Acosta and Padilla Lozoya 2021) that affected the area: From 1954 to 1956, the Florence hurricanes, Gladys, Hilda, Janet, and Anna along with a tropical storm (Dora); from 1988 to 1990, hurricanes Debby and Diana; and from 2005 to 2007, hurricanes José, Stan, Dean, Lorenzo, and Marco. This is like what has happened in other coastal areas where the impact of extreme and concurrent climate events has been recorded (Ruiz-Fernández et al. 2007; Eyrolle et al. 2012; Rajan et al. 2013; Olvera Prado 2014; Botello et al. 2018; Irabien et al. 2019; Dellapenna et al. 2022). Understanding historical trends in climate events is crucial to understanding ecosystem dynamics. The two sediment profiles exhibited fluctuations in PTM concentrations. The maximum concentrations in both profiles may be linked to meteorological occurrences in the Mexican region during the dates in which these peaks were measured; the high levels of precipitation in these systems provide a greater sediment load from the high areas of the Papaloapan Basin towards the coastal plain of Veracruz where the Alvarado Lagoon System is located. The Alvarado Lagoon is the largest in the area and directly receives the discharges of the Papaloapan, Blanco, and Acula rivers, as well as influences from the Gulf of Mexico through the entrance of the lagoon’s inlet. Understanding the historical climate trends and meteorological events is crucial to comprehending the dynamics of ecosystems. This information allows us to analyze their variations, vulnerability, sedimentation rates, and resilience. Real estate development in the Papaloapan Basin and the coastal area of Puerto Alvarado may cause an increase in anthropogenic contributions to the Alvarado Lagoon System. These effects include a possible natural imbalance of the coastal system, interference in sediment transport and alteration of the cycles of biological organisms. The EF enables differentiation between metals of anthropogenic and/or natural origin and facilitated the assessment of their impact. Values ranking from 0.5 to 1.5 imply that the metals are likely entirely from the Earth’s crust or natural weathering processes, while a value exceeding 1.5 indicates that some of the material may be contributed by a source to the earth’s crust or by anthropogenic sources (Zhang and Liu 2002; Armstrong-Altrin and Pineda-Natalhy 2014; Vetrimurugan et al. 2019; Armstrong-Altrin et al. 2019; Gargouri et al. 2023). The EF < 1 results for PTMs in both sedimentary profiles, suggest that the sediments have no experienced enrichment, and that they were derived from the parent material. This finding is consistent with previous studies (Ruiz-Fernández et al. 2014; Botello et al. 2018; Velandia Aquino et al. 2018; Mapel-Hernández et al. 2021; Velandia-Aquino et al. 2023), and indicates that the sediments contain detritus and weathered rocks, from soils that have undergone diagenetic changes. These sediments also possess a small number of anthropogenic metals. Regarding the Geoaccumulation Index, the analysis shows that the sedimentary columns in both profiles are uncontaminated by PTMs, indicated that the presence of these metals in the sediments of lagoon are lithogenic origin. Moreover, the concentrations of the PTMs analyzed were found to be below the ERL and ERM thresholds of the NOAA Sedimentary Quality Guidelines (Buchman 2008). Therefore, it can be concluded that there is no risk of impacting benthic communities.
Content of Polycyclic Aromatic Hydrocarbons (PAHs)
In both sediment profiles, Phe was the PAH with the higher concentration, this is expected, as it has greater environmental persistence than Nap. Both Nap and Phe are low molecular weight PAHs (containing two and three benzene rings), are highly degradable in the water column through processes as photooxidation, chemical oxidation, and biological transformation (Billur Balcioglu 2016; Sarma et al. 2016), as long as they do no attach to humic compounds that carry them to their deposition in sediments. The compounds’ semi-volatile nature and low concentrations, suggest three possible origins: (i) petrogenic results from the incomplete combustion (100–300 °C) of biomass produced in the preparation of nearby agricultural soils, as well as incidental spills from small fishing vessels in more recent times (Ravindra et al. 2008); (ii) potential biogenesis due to very small quantities of both PAHs and minimal temporal variation (Tobiszewski and Namiesnik 2012; Stogiannidis and Laane 2015). This indicates a related breakdown of organic matter in tropical regions (Krauss et al. 2005; Pang et al. 2021); (iii) the adsorption dynamics and thermodependent desorption of Nap and Phe, in tropical environments, where temperatures greater than 25 °C, lead to frequent desorption of organic fraction PAHs either adhere back to other organic particles or solubilize (Hiller et al. 2008; Achour et al. 2023). Studies have shown that the most dominant PAHs on the soil surface are those with molecules with 2 or 3 rings (likewise Nap and Phe). These PAHs have been found to reach coastal areas via runoff as reported by Guo et al. (2011), and Melnyk et al. (2015). Additionally, Nap and Phe are PAHs have been identified as components of crude oil and derived fuels in previous studies (Abdullah et al. 2021; Goto et al. 2021; Huynh et al. 2021; Stepanova et al. 2022). The low levels of PAHs make it impossible to stablish a correlation between their presence due to any specific hydrometeorological event or specific environmental process in the studied years.
The ecotoxicological risk posed by the PAHs identified in the ecosystem is considered negligible as they are three orders of magnitude below the sedimentary quality standards (Buchman 2008).
The accumulations of both groups of pollutants, PTMs and PAHs have garnered attention primarily in impacted by oil exploitation, processing, and distribution. It has been observed that the presence of hydrocarbons also contributes to elevated concentrations of heavy metals due to the refining and distillation of crude oil as one of the most important causes of pollution by PAHs (Velandia Aquino 2010; Sarma et al. 2016; Bojórquez Sánchez et al. 2018; Valentina et al. 2021; Velandia-Aquino et al. 2023). It was anticipated that a correlation would exist between the levels of PTMs and PAHs in the sediments of Alvarado Lagoon due to the shared origin of both pollutants and their potential hazard of causing synergistic effects in ecosystems and biota at the metabolic and cellular level (Saeedi et al. 2020; Bandowe et al. 2021; Valentina et al. 2021; Lu et al. 2023).
The study confirmed that PTMs non-biodegradability resulted in their longer retention in the sediment in both profiles, while PAHs were mostly absent. On the other hand, it is also important to recognize that planktonic organisms and microplastics transport PAHs playing a crucial role potentially reduce them in sediments and initiating their biotransformation (Fan and Reinfelder 2003; Berrojalbiz et al. 2009; Fu et al. 2018; Wang et al. 2019; Achour et al. 2023). In addition to the above, the flow rates of the Blanco and Papaloapan Rivers (S1) allow some of the pollutants that enter the Alvarado Lagoon to be exported to the Gulf of Mexico. This results in a lower accumulation of pollutants in the sedimentary profiles, as demonstrated in this study. Nevertheless, the resilience could be altered if there are modifications in land uses change and unregular industrial activities that directly affect the ecosystem.
Statistical Analysis
The results of the correlation of Pearson, Cluster, Factor Analysis, and PCA, in all showed a marked difference between PTMs and PAHs, putting them in two separated groups, indicating a common origin among themselves, but at the same time, a different source in the sediments of this ecosystem, so it is concluded that PTMs are lithogenic and PAHs are petrogenic. For both types of contaminants, similar distribution patterns were observed throughout the sedimentary column. The high correlations that occurred between the metals Ni, Cr, V, and slightly Pb with Al are largely associated with geogenic or lithogenic sources, probably with similar biogeochemical pathways for accumulation in the sediments of the system, and this is confirmed by grouping observed in the PCA (Yuan et al. 2011; Al-Mur et al. 2017), and have successfully used correlation and PCA to distinguish the sources of metals in sediments from the Red Sea in Saudi Arabia, and Lake Taihu in China, respectively. The enrichment factor showed that the sediments are not enriched by any of the PTMs and the Igeo indicated that there is no contamination by these metals, thus, the source of the PTMs is lithogenic.
Conclusions
The concentrations of potentially toxic metals are low and are associated with a natural source, no contribution of anthropogenic metal was observed. Both profiles presented periodic oscillations of PTMs and maxima related to meteorological events that have affected the area, while the amounts of PAHs are very low and petrogenic, so that the levels of both pollutants do not represent a sedimentary or ecotoxicological risk, indicating the resilience of this ecosystem. No relationship was observed between PTMs and PAHs in terms of origin or similar distribution in sedimentary layers. Urban and industrial development may accelerate the degradation of the current environment and can jeopardize the sustainability of this ecosystem if management programs are not established for fossil fuel exploration and prospecting activities and to regulate wastewater discharges that alter the environmental conditions, and ecosystem services on which an important part of the region’s population depends.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
Data will be made available on request.
References
Abdullah, E., M. Ebiad, A. Rashad, S. El Nady, M. El-Sabbagh. 2021. Thermal maturity assessment of some Egyptian crude oils as implication from naphtalene, phenanthrene, and alkyl substituents. Egyptian Journal of Petroleum 30 (1): 17–24. https://doi.org/10.1016/j.ejpe.2020.12.004.
Achour, D., M. Trifi, R. Azouri, A. Charef, and S. Rokbani. 2023. Highlighting the protective role of coastal rivers: Dynamics of adsorption and desorption of naphthalene and pyrene of Lebna River sediments (N-E Tunisia). Water, Air, and Soil Pollution 234: 4. https://doi.org/10.1007/s11270-022-06002-z.
Algül, F., and M. Beyhan. 2020. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Scientific Reports 10: 11782. https://doi.org/10.1038/s41598-020-68833-2.
Al-Mur, B., A. Quicksall, and A. Al-Ansari. 2017. Spatial and temporal distribution of heavy metals in coastal core sediments from the Red Sea, Saudi Arabia. Oceanologia 59: 262–270. https://doi.org/10.1016/j.oceano.2017.03.003.
Armstrong-Altrin, J., and O. Pineda-Natalhy. 2014. Microtextures of detrital sand grains from the Tecolutla, Nautla, and Veracruz beaches, western Gulf of Mexico, Mexico: Implications for depositional environment and paleoclimate. Arabian Journal of Geosciences 7 (10): 4321–4333. https://doi.org/10.1007/s12517-013-1088-x.
Armstrong-Altrin, J., A. Botello, S. Villanueva, and L. Soto. 2019. Geochemistry of surface sediments from the northwestern Gulf of Mexico: Implications for provenance and heavy metal contamination. Geological Quarterly 63 (3): 522–538. https://doi.org/10.7306/gq.1484.
Balcioglu, E.B. 2016. Potential effects of polycyclic aromatic hydrocarbons (PAHs) in marine foods on human health: A critical review. Toxin Reviews 35 (3–4): 98–105. https://doi.org/10.1080/15569543.2016.1201513.
Balgobin, A., and N. Ramroop. 2019. Source apportionment and seasonal cancer risk of polycyclic aromatic hydrocarbons of sediments in a multi-use coastal environment containing a Ramsar wetland, for a Caribbean island. Science of the Total Environment 664: 474–486. https://doi.org/10.1016/j.scitotenv.2019.02.031.
Bandowe, B., N. Shukurov, S. Leimer, M. Kersten, Y. Steinberger, and W. Wilcke. 2021. Polycyclic aromatic hydrocarbons (PAHs) in soils of an industrial area in semi-arid Uzbekistan: Spatial distribution, relationship with trace metals and risk assessment. Environmental Geochemistry and Health 43 (11): 4847–4861. https://doi.org/10.1007/s10653-021-00974-3.
Bastakoti, U., J. Robertson, C. Bourgeois, C. Marchand, and A. Alfaro. 2019. Temporal variations of trace metals and a metalloid in temperate estuarine mangrove sediments. Environmental Monitoring and Assessment 191: 780. https://doi.org/10.1007/s10661-019-7916-z.
Berrojalbiz, N., S. Lacorte, A. Calbet, E. Saiz, C. Barata, and J. Dachs. 2009. Accumulation and cycling of polycyclic aromatic hydrocarbons in zooplankton. Environmental Science and Technology 43: 2295–2301. https://doi.org/10.1021/es8018226.
Bojórquez Sánchez, S., A.J. Marmolejo Rodríguez, A.C. Ruiz Fernández, A. Sánchez González, J.A. Sánchez Cabeza, H. Bojórquez Leyva, and L.H. Pérez Bernal. 2018. Nickel and vanadium natural enrichment in sediment cores near oil extraction sites in the Gulf of México. International Review Contacto Ambiente 34 (4): 713–723. https://doi.org/10.20937/rica.2018.34.04.12.
Botello, A., J. Armstrong Altrin, S. Villanueva, L. Velandia, and A. Arellano. 2022. Evaluation of potentially toxic metal concentrations in the Terminos Lagoon, Campeche, Gulf of Mexico. Carpathian Journal of Earth And Environmental Sciences 17 (2): 245–258. https://doi.org/10.26471/cjees/2022/017/218.
Botello, A.V., F.S. Villanueva, A.L. Velandia, G.E. de la Lanza, and R.F. Rivera. 2018. Analysis and tendencies of metals and POPs in a sediment core from the Alvarado Lagoon System (ALS), Veracruz, México. Archives of Environmental Contamination and Toxicology 75: 157–173. https://doi.org/10.1007/s00244-018-0516-z.
Buchman, M. 2008. Screening Quick Reference Tables (SQuiRTs), Report 08–1. National Oceanic and Atmospheric Administration NOAA), Office of Response and Restoration Division, Seattle WA. https://repository.library.noaa.gov/view/noaa/9327.
CONABIO. 1998. Regiones Prioritarias Marinas de México. Comisión Nacional para el Conocimiento y uso de la Biodiversidad. Recover November 6, 2022, in http://www.conabio.gob.mx/conocimiento/manglares/doctos/caracterizacion/GM53_Sistema_Lagunar_de_Alvarado_veracruz_caracterizacion.pdf.
Cornelissen, G., G. Breedveld, S. Kalaitzidis, K. Christanis, A. Kibsgaard, and A. Oen. 2006. Strong sorption of native PAHs to pyrogenic and unburned carbonaceous geosorbents in sediments. Environmental Science and Technology 40: 1197–1203. https://doi.org/10.1021/es0520722.
Dalia, M., A. Salem, A. Khaled, A. El Nemr, and A. El-Sikaily. 2014. Comprehensive risk assessment of heavy metals in surface sediments along the Egyptian Red Sea coast. Egyptian Journal of Aquatic Research 40 (4): 349–362. https://doi.org/10.1016/j.ejar.2014.11.004.
Dellapenna, T., C. Hoelscher, L. Hill, L. Critides, B.V. Salgado, M. Bell, E. Al Mukaimi, J. Du, K. Park, and A. Knap. 2022. Hurricane harvey delivered a massive load of mercury-rich sediment to Galveston Bay, TX, USA. Estuaries and Coasts 45: 428–444. https://doi.org/10.1007/s12237-021-00990-7.
Di Duca, F., P. Mountuori, U. Trama, A. Masucci, G.M. Borrelli, and M. Triassi. 2023. Health risk assessment of PAHs from estuarine sediments in the South of Italy. Toxics 11 (2): 172. https://doi.org/10.3390/toxics11020172.
Estevez, E., M. Cabrera, J. Fernández-Vera, A. Molina-Díaz, J. Robles-Molina, and M. Palacios-Díaz. 2016. Monitoring priority substances, other organic contaminants, and heavy metals in a volcanic aquifer from different sources and hydrological processes. Science of the Total Environment 551–552: 186–196. https://doi.org/10.1016/j.scitotenv.2016.01.177.
Eyrolle, F., O. Radakovich, P. Raimbault, S. Charmasson, Ch. Antonelli, E. Ferrand, D. Aubert, G. Raccasi, S. Jacquet, and R. Gurriaran. 2012. Consequences of hydrological events on the delivery of suspended sediment and associated radionuclides from the Rhône River to the Mediterranean Sea. Journal of Soils and Sediments 12: 1479–1495. https://doi.org/10.1007/s11368-012-0575-0.
Fan, C., and J. Reinfelder. 2003. Phenanthrene accumulation kinetics in marine diatoms. Environmental Science and Technology 37 (15): 3405–3412. https://doi.org/10.1021/es026367g.
Förstner, U., and G. Wittmann. 1979. Metal pollution in aquatic environment, 475. Berlin: Springer-Verlag.
Fu, W., M. Xu, L. Hu, W. Cai, C. Dai, and Y. Jia. 2018. Biodegradation of phenanthrene by endophytic fungus Phomopsis liquidambari in vitro and in vivo. Chemosphere 203: 160–169. https://doi.org/10.1016/j.chemosphere.2018.03.164.
García Acosta, V., and R. Padilla Lozoya. 2021. Historia y memoria de los huracanes y otros episodios hidrometeorológicos extremos en México. Cinco siglos: del año 5 pedernal a Janet (1° ed.). Xalapa, Veracruz: Universidad Veracruzana. Recover April 6, 2022, in https://portal.ucol.mx/content/micrositios/325/file/AnexosHuracan.pdf.
Gargouri D., M. Gzam, and H. Abida. 2023. Effect of anthropogenic activity on the levels of metals in surface sediments of the Gabes Gulf (Tunisia). Research Square. https://doi.org/10.21203/rs.3.rs-2801202/v1.
Goto, Y., K. Nakamuta, and H. Nakata. 2021. Parent and alkylated PAHs profiles in 11 petroleum fuels and lubricants: Applications for oil spill accidents in the environment. Ecotoxicology and Environmental Safety 224: 112644. https://doi.org/10.1016/j.ecoenv.2021.112644.
Guentzel, J., E. Portilla, K. Keith, and E. Keith. 2011. The Alvarado Lagoon- environment, impact, and conservation. In F. G. A, Lagoons: biology, management, and environmental impact. Nova Science Publishers Inc. 397–415.
Guo, J., F. Wu, L. Znag, H. Liao, R. Zhang, W. Li, X. Zhao, S. Chen, and B. Mai. 2011. Screening level of PAHs in sediment core from Lake Hongfeng, Southwest China. Archives of Environmental Contamination and Toxicology 60: 590–596. https://doi.org/10.1007/s00244-010-9568-4.
Hiller, E., L. Jurkovič, and M. Bartaľ. 2008. Effect of temperature on the distribution of polycyclic aromatic hydrocarbons in soil and sediment. Soil and Water Research 3 (4): 231–240. https://doi.org/10.17221/28/2008-SWR.
Hung, J., and C. Hsu. 2004. Present state and historical changes of trace metal pollution in Kaoping coastal sediments, southwestern Taiwan. Marine Pollution Bulletin 49 (11–12): 986–998. https://doi.org/10.1016/j.marpolbul.2004.06.028.
Huynh, K., E. Jensen, and J. Sundberg. 2021. Extended characterization of petroleum aromatics using off-line LC-GC-MS. PeerJ Analytical Chemistry 3: e12. https://doi.org/10.7717/peerj-achem.12.
Hylland, K. 2006. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. Journal of Toxicology and Environment Health 69 (1–2): 109–123. https://doi.org/10.1080/15287390500259327.
IAEA. 2009. Aplicación de técnicas nucleares en la solución de problemas específicos del manejo integrados de zonas costeras en el Gran Caribe. Viena: IAEA.
Irabien, M., A. Cearreta, J. Gómez-Arozamena, H. Serrano, J. Sánchez-Cabeza, and A.C. Ruiz-Fernández. 2019. Geological record of extreme floods and anthropogenic impacts on an industrialised bay: The inner Abra of Bilbao (northern Spain). Science of the Total Environment 696: 133946. https://doi.org/10.1016/j.scitotenv.2019.133946.
Krauss, M., W. Wilcke, C. Martius, A.G. Bandeira, M.V. Garcia, and W. Amelung. 2005. Atmospheric versus biological sources of polycyclic aromatic hydrocarbons (PAHs) in a tropical rain forest environment. Environmental Pollution 135: 143–154. https://doi.org/10.1016/j.envpol.2004.09.012.
Lacoste, E., A. Jones, M. Callier, J. Klein, F. Lagarde, and V. Derolez. 2023. A review of knowledge on the impacts of multiple anthropogenic pressures on the soft-bottom benthic ecosystem in Mediterranean Coastal Lagoons. Estuaries and Coasts. 46: 2190–2207 https://doi.org/10.1007/s12237-023-01188-9.
Li, Z., X. Li, S. Wang, F. Che, Y. Zhang, P. Yang, J. Zhang, Y. Liu, H. Guo, and Z. Fu. 2023. Adsorption and desorption of heavy metals at water sediment interface based on Bayesian model. Journal of Environmental Management 329: 117035. https://doi.org/10.1016/j.jenvman.2022.117035.
Liu, X., Z. Chen, J. Wu, Z. Cui, and P. Su. 2020. Sedimentary polycyclic aromatic hydrocarbons (PAHs) along the mouth bar of the Yangtze River Estuary: Source, distribution, and potential toxicity. Marine Pollution Bulletin 159: 111494. https://doi.org/10.1016/j.marpolbul.2020.111494.
Lu, Y., Y. Zhang, X. Xiang, S. Zhang, and H. Yao. 2023. Combined pollution of heavy metals and polycyclic aromatic hydrocarbons in the soil in Shenfu Region, China: A case of three different cities. Environmental Monitoring and Assessment 195 (1): 167. https://doi.org/10.1007/s10661-022-10747-9.
Mallén, R.C., P.X. Esparza, G.T. Vite, V.N. Núnez, A.A. Barrena, H.M. Garay, and H.M. Sánchez. 2006. Diagnóstico Ambiental y Forestal del Estado de Veracruz. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias; Comisión Nacional Forestal; Organización Internacional de Maderas Tropicales. Recover January 10, 2023, in http://www.itto.int/files/itto_project_db_input/2576/Technical/DIAGNOSTICO%20AMBIENTAL%20Y%20FORESTAL%20DEL%20ESTADO%20DE%20VERACRUZ.pdf.
Mapel-Hernández, M., J. Armstrong-Altrin, A. Botello, and F. Lango-Reynoso. 2021. Bioavailability of Cd and Pb in sediments of the National Park Veracruz. Applied Geochemistry 133: 105085. https://doi.org/10.1016/j.apgeochem.2021.105085.
Melnyk, A., A. Detlaff, J. Kuklińska, J. Namieśnik, and L. Wolska. 2015. Concentration and sources of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in surface soil near a municipal solid waste (MSW) landfill. Science of the Total Environment 530–531: 18–27. https://doi.org/10.1016/j.scitotenv.2015.05.092.
Mihankhah, T., M. Saeedi, and A. Karbassi. 2020. Contamination and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban dust from different land uses in the most populated city of Iran. Ecotoxicology and Environmental Safety 187: 109838. https://doi.org/10.1016/j.ecoenv.2019.109838.
Milligan, T., and B. Law. 2013. Contaminants at the sediment−water interface: Implications for environmental impact assessment and effects monitoring. Environmental Science and Technology 47 (11): 5828–5834. https://doi.org/10.1021/es3031352.
Montuori, P., E. De Rosa, F. Di Duca, B. De Simone, S. Scippa, I. Russo, P. Sarnacchiaro, and M. Triassi. 2022. Polycyclic aromatic hydrocarbons (PAHs) in the dissolved phase, particulate matter, and sediment of the Sele River, Southern Italy: A focus on distribution, risk assessment, and sources. Toxics 10 (7): 401. https://doi.org/10.3390/toxics10070401.
Mountouris, A., E. Voutsas, and D. Tassios. 2002. Bioconcentration of heavy metals in aquatic environments the importance of bioavailability. Marine Pollution Bulletin 44 (10): 1136–1141. https://doi.org/10.1016/S0025-326X(02)00168-6.
Müller, G. 1979. Schwermetalle in den sedimenten des Rheins-Veränderungen seit 1971. Umschau 79: 778–783.
Nava-Tablada, M.E.N., and J.D.J.V. Villanueva-Fortanelli. 2021. Contexto socioeconómico y problemática ambiental de la pesca en el Sistema Lagunar de Alvarado, Veracruz, desde la perspectiva de los pobladores. Revista de El Colegio de San Luis XI (22): 1–30. https://doi.org/10.21696/rcsl102120201179.
Neves, P., F. Santos, L. Araújo, S. Taniguchi, P. Ferreira, R. Figueira, R.A. Lourenco, and M. Bícego. 2022. Unusual natural polycyclic aromatic hydrocarbons in sediment cores of an Amazon estuary. Marine Pollution Bulletin 183: 114059. https://doi.org/10.1016/j.marpolbul.2022.114059.
Nguyen, T., P. Loganathan, T. Nguyen, S. Vignerwaran, J. Kandasamy, D. Slee, G. Stevenson, and R. Naidu. 2014. Polycyclic aromatic hydrocarbons in road-deposited sediments, water sediments, and soils in Sydney, Australia: Comparisons of concentration distribution, sources and potential toxicity. Ecotoxicology and Environmental Safety 104: 339–348. https://doi.org/10.1016/j.ecoenv.2014.03.010.
Olvera Prado, E. 2014. Respuesta hidrodinámica de las lagunas y ríos del estuario del Papaloapan un forzamiento meteorológico. México: Tesis de Maestría en Ciencias de la Tierra, UNAM.
Ontiveros-Cuadras, J., C. Ruiz-Fernández, J. Sánchez-Cabeza, J. Sericano, H. Pérez-Bernal, F. Páez-Osuna, R.B. Dunbar, and D. Mucciarone. 2019. Recent history of persistent organic pollutants (PAHs, PCBs, PBDEs) in sediments from a large tropical lake. Journal of Hazardous Materials 368: 264–273. https://doi.org/10.1016/j.jhazmat.2018.11.010.
Pang, S., S. Suratman, Y. Hii, B. Simoneit, and N. Tahir. 2021. Sources of polycyclic aromatic hydrocarbons in South China Sea short core sediments off southern part Terengganu, Malaysia and multivariate statistics approaches. Sains Malaysiana 50 (9): 2511–2522. https://doi.org/10.17576/jsm-2021-5009-02.
Ponce-Vélez, G., and G. de la Lanza-Espino. 2019. Organophosphate pesticides in Coastal Lagoon of the Gulf of Mexico. Journal of Environmental Protection 10 (2): 103–117. https://doi.org/10.4236/jep.2019.102007.
Praavena, S.M., M. Radojevic, M.H. Abdullah, and A.Z. Aris. 2008. Application of sediment quality guidelines in the assessment of mangrove surface sediment in Mengkabong Lagoon, Sabah. Malaysia. J. Environ. Health. Sci. Eng. 5 (1): 35–42.
Qasem, N., R. Mohammed, and D. Lawal. 2021. Removal of heavy metal ions from wastewater: a comprehensive and critical review. npj Clean Water 4: 36. https://doi.org/10.1038/s41545-021-00127-0.
Rafiei, B., F. Ahmadi-Ghomi, A. Seif, et al. 2023. Contamination assessment and spatial distribution of heavy metals in the Sefidrud Delta coastal lagoons, Caspian Sea, N Iran. Environmental Monitoring and Assessment 195: 464. https://doi-org.pbidi.unam.mx:2443/10.1007/s10661-023-11096-x.
Rajan, R., L. Ramanathan, G. Singh, and S. Chidambaram. 2008. Assessment of metal enrichments in tsunamigenic sediments of Pichavaram mangroves, south-east coast of India after the tsunami of 2004. Environmental Monitoring and Assessment 147: 389–411. https://doi.org/10.1007/s10661-007-0128-y.
Rajan, R., G. Singh, J. Routh, and A. Ramanathan. 2013. Trace metal fractionation in the Pichavaram mangrove–estuarine sediments in southeast India after the tsunami of 2004. Environmental Monitoring and Assessment 185: 8197–8216. https://doi.org/10.1007/s10661-013-3167-6.
Ravindra, K., R. Sokhi, and Grieken., R. 2008. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment 42 (13): 2895–2921. https://doi.org/10.1016/j.atmosenv.2007.12.010.
Ridgway, J., and G. Shimmeld. 2002. Estuaries as repositories of historical contamination and their impact on shelf seas. Estuarine, Coastal and Shelf Science 55: 903–928. https://doi.org/10.1006/ecss.2002.1035.
Rudnick, R., and S. Gao. 2003. Composition of the continental crust. Treatise on Geochemistry 3: 1–64. https://doi.org/10.1016/b0-08-043751-6/03016-4.
Ruiz-Fernández, A., C. Hillaire Marcel, F. Páez Osuna, B. Ghaleb, and M. Caballero. 2007. 210Pb chronology and trace metal geochemistry at Los Tuxtlas, Mexico, as evidenced by a sedimentary record from the Lago Verde crater lake. Quaternary Research 67: 181–192. https://doi.org/10.1016/j.yqres.2006.11.003.
Ruiz-Fernández, A., and F. Páez-Osuna. 2011. Cronómetros radioactivos de vida corta (210Pb, 137Cs) en el estudio de los cambios ambientales. In G. Argaez, and P. L. Rosales, Interacciones en el Planeta Tierra. 63–72. México: ICMyL, UNAM.
Ruiz-Fernández, A.C., J.A. Sánchez Cabeza, C. Alonso-Hernández, V. Martínez-Herrera, L.H. Pérez-Bernal, M. Preda, C. Hillaire-Marcel, J. Gastaud, and A.J. Quejido-Cabezas. 2012. Effects of land use change and sediment mobilization on coastal contamination (Coatzacoalcos River, Mexico). Continental Shelf Research 37: 57–65. https://doi.org/10.1016/j.csr.2012.02.005.
Ruiz-Fernández, A., M. Maanan, J. Sánchez-Cabeza, L. Pérez-Bernal, P. López-Mendoza, and A. Limoges. 2014. Chronology of recent sedimentation and geochemical characteristics of sediments in Alvarado Lagoon, Veracruz (southwestern Gulf of México). Ciencias Marinas 40 (4): 291–303. https://doi.org/10.7773/cm.v40i4.2473.
Ruiz-Fernández, A., J. Sánchez-Cabeza, L. Pérez-Bernal, M. Blaauw, J. Cardoso-Mohedano, M. Aquino-López, and S. Giralt. 2022. Recent trace element contamination in a rural crater lake, NW Mexico. Journal of Paleolimnology 69:191–212. https://doi.org/10.1007/s10933-022-00268-3.
Saeedi, M., L. Grace, and J. Li. 2020. Effects on co-existing heavy metals and natural organic matter on sorption/desorption of polycyclic aromatic hydrocarbons in soils: a review. Pollution 6 (1): 1–24. https://doi.org/10.22059/poll.2019.284335.638.
Sarma, H., N. Islam, P. Borgohain, A. Sarma, and M. Prasad. 2016. Localization of polycyclic aromatic hydrocarbons and heavy metals in surface soil of Asia’s oldest oil and gas drilling site in Assam, northeast India: Implications for the bio-economy. Emerging Contaminants 2: 119–127. https://doi.org/10.1016/j.emcon.2016.05.004.
Seelen, E., G. Massey, and R. Mason. 2018. The role of sediment resuspension on estuarine suspended particulate mercury dynamics. Environmental Science and Technology 52 (14): 7736–7744. https://doi.org/10.1021/acs.est.8b01920.
Shi, C., H. He, Z. Xia, H. Gan, Q. Xue, Z. Cui, and J. Chen. 2022. Heavy metals and Pb isotopes in a marine sediment core record environmental changes and anthropogenic activities in the Pearl River Delta over a century. Science of the Total Environment 814: 151934. https://doi.org/10.1016/j.scitotenv.2021.151934.
Stepanova, A., E. Gladkov, E. Osipova, O. Gladkova, and O.V. Tereshonok. 2022. Biorremediation of soil from petroleum contamination. Processes 10 (1224). https://doi.org/10.3390/pr10061224.
Stogiannidis, E., and R. Laane. 2015. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. In Reviews of environmental contamination and toxicology, ed. D. Whitacre, 234: 50–133. Springer. https://doi.org/10.1007/978-3-319-10638-0_2.
Suman, S., A. Sinha, and A. Tarafdar. 2016. Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Science of the Total Environment 545–546: 353–360. https://doi.org/10.1016/j.scitotenv.2015.12.061.
Sutilli, M., T. Combi, G. Domingues, and M., and Martins, C. 2020. One century of historical deposition and flux of hydrocarbons in a sediment core from a South Atlantic RAMSAR subtropica estuary. Science of the Total Environment 706: 136017. https://doi.org/10.1016/j.scitotenv.2019.136017.
Tobiszewski, M., and Namiesnik., J. 2012. PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution 162: 110–119. https://doi.org/10.1016/j.envpol.2011.10.025.
USEPA. 2009. Ecological toxicity information. Recover November 11, 2022, in http://www.epa.gov/region5superfund/ecology/html/toxprofiles.htm.
Valentina, P., M. Mistri, T.M. Granata, L. Moruzzi, L. Meloni, F. Massara, A. Sfriso, A.A. Sfriso, and C. Munari. 2021. Sediment contamination by heavy metals and PAH in the Piombino Channel (Tyrrhenian Sea). Water 13 (11): 1487. https://doi.org/10.3390/w13111487.
Vázquez, L., Z. Rodríguez, and G. Ramírez. 2009. Caracterización del sitio de manglar Sistema Lagunar de Alvarado Veracruz, en Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Sitios de manglar con relevancia biológica y con necesidades de rehabilitación ecológica. CONABIO.
Velandia Aquino, L. B. 2010. Determinación de la contaminación por metales en el Complejo Lagunar del Istmo de Tehuantepec, Oaxaca, México. MSc thesis. Cd. México, México: Universidad Nacional Autónoma de México.
Velandia Aquino, L.B., A. Botello, and F. Villanueva. 2018. Chronological study of metals in a sediment core from tle Alvarado Lagoon System (SLA), Veracruz, México. Proceedings 6th International Symposium on Sediment Management In: Revista Internacional de Contaminación Ambiental 34: 329–334. https://doi.org/10.20937/2018.34.M6ISSM.
Velandia-Aquino, L., A. Botello, G. Ponce-Vélez, J. Armstrong-Altrin, A. Ruiz-Fernández, B. Prado, and S. Villanueva-Fragoso. 2023. 210Pb geochronology and metal concentrations in sediment cores recovered in the Alvarado Lagoon System, Veracruz, Mexico. Chemosphere 330: 138709. https://doi.org/10.1016/j.chemosphere.2023.138709.
Vetrimurugan, E., V. Shruti, M. Jonathan, D. Priyadarsi, S. Sarkar, B. Rawlins, and L. Campos Villegas. 2019. Comprehensive study on metal contents and their ecological risks in beach sediments of KwaZulu-Natal province, South Africa. Marine Pollution Bulletin 149: 110555. https://doi.org/10.1016/j.marpolbul.2019.110555
Villas-Hernández, B., S. Pérez-Elizalde, P. Rodríguez-Trejo, and D. Pérez-Rodríguez. 2022. Análisis espacio temporal de la ocurrencia de incendios forestales en el estado mexicano de Oaxaca. Revista Mexicana de Ciencias Forestales 13: 74. https://doi.org/10.29298/rmcf.v13i74.1274.
Wang, J., X. Liu, G. Liu, Z. Zhang, H. Wu, B. Cui, J. Bai, and W. Zhang. 2019. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicology and Environmental Safety 173: 331–338. https://doi.org/10.1016/j.ecoenv.2019.02.037.
Wang, R., D. Xu, and Q. Ge. 2020. Modern modes of sediment distribution and the anthropogenic heavy metal pollution record in northeastern Beibu Gulf, south China sea. Marine Pollution Bulletin 150: 110694. https://doi.org/10.1016/j.marpolbul.2019.110694.
WHO. 2003. Polynuclear aromatic hydrocarbons in drinking water. Background document for development of WHO Guidelines for Drinking-water Quality [WHO/SDE/WSH/03.04/59]. En In: Guidelines for drinking-water quality (2° ed., Vol. 2). Genova. Reecover november 2, 2022, in https://www.who.int/docs/default-source/wash-documents/wash-chemicals/polynuclear-aromatic-hydrocarbons-background-document.pdf.
Xiao, H., A. Shahab, B. Xi, Q. Chang, S. You, J. Li, X. Sun, H. Hang, and X. Li. 2021. Heavy metal pollution, ecological risk, spatial distribution, and source identification in sediments of the Lijiang River, China. Environmental Pollution 269 (116189): 1–10. https://doi.org/10.1016/j.envpol.2020.116189.
Yuan, H., J. Shen, E. Liu, J. Wang, and X. Meng. 2011. Assessment of nutrients and heavy metals enrichment in surface sediments from Taihu Lake, a eutrophic shallow lake in China. Environmental Geochemistry and Health 33: 67–81. https://doi.org/10.1007/s10653-010-9323-9.
Yuan, Y., Q. Wang, X. Dong, Y. Zhu, Z. Wu, Q. Yang, Y. Zuo, S. Liang, C. Wang, and X. Zhu. 2022. In situ, high-resolution evidence of metals at the sediment-water interface under ice cover in a seasonal freezing lake. Frontiers in Ecology and Evolution 10: 956903. https://doi.org/10.3389/fevo.2022.956903.
Yunker, M., R. Macdonald, P. Ross, S. Johannessen, and N. Dangerfield. 2015. Alkane and PAH provenance and potential bioavailability in coastal marine sediments subject to a gradient of anthropogenic sources in British Columbia, Canada. Organic Geochemistry 89–90: 80–116. https://doi.org/10.1016/j.orggeochem.2015.10.002.
Zhang, J., and C. Liu. 2002. Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science 54 (6): 1051–1070. https://doi.org/10.1006/ecss.2001.0879.
Zhao, Z., Z. Qin, J. Cao, and L. Xia. 2017. Source and ecological risk characteristics of PAHs in sediments from Qinhuai River and Xuanwu Lake, Nanjing, China. Journal of Chemistry 2017: 1–18. https://doi.org/10.1155/2017/3510796.
Acknowledgements
We appreciate the support of Dr. Omar Amador Muñoz of the Laboratory of Chemical Speciation of Atmospheric Organic Aerosols of the Institute of Atmospheric Sciences and Climate Change of the UNAM.
Funding
This research was funded by the National Autonomous University of Mexico (UNAM). National Council of Science and Technology (CONACYT) provided the Ph.D. fellowship to Velandia-Aquino LB (N° 449333).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wen-Xiong Wang
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velandia-Aquino, L.B., Botello, A.V., Ponce-Vélez, G. et al. Vertical Distribution of Potentially Toxic Metals and PAHs in the Alvarado Lagoon, Veracruz in the Southern Gulf of Mexico. Estuaries and Coasts (2023). https://doi.org/10.1007/s12237-023-01307-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-023-01307-6