Abstract
Quantifying trophic relationships within and between species in terms of trophic position, ontogeny and trophic diversity provides information on community-level structure and function. Little attention has been focused on examining the trophic structure and temporal changes of communities in freshwater-marine coupled systems in the subarctic and associated impacts of anthropogenic activity on trophic interactions. Therefore, the objective of this study was to quantify the trophic position of mobile consumers (15 species in total) within the lower Churchill River area (Churchill, Manitoba, Canada), examine trophic position variation in relation to ontogeny, and measure the trophic diversity of a fish assemblage before (1993–1995) and after (2019–2020) the installation of the Churchill River weir in the late 1990s. We used stable isotopes (δ13C and δ15N) to quantify individual and group-level variation in trophic position of thirteen fish species and two seal species and also assessed six community-level metrics of a three fish species assemblage between time periods. Overall, species that mainly foraged on freshwater resources occupied lower trophic positions than species that mainly consumed marine resources. Trophic position increased with fish age only in cisco, fourhorn sculpin, Greenland cod and northern pike. A temporal shift from a trophically diverse to a more trophically redundant fish assemblage occurred between 1993–1995 and 2019–2020. As a result, these predator species now play similar trophic roles. Information on the long-term change in trophic structure of this sub-Arctic estuarine system may help with understanding how anthropogenic activity and climate change have influenced the trophic diversity of a fish assemblage inhabiting this system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The movement of nutrients, prey and consumers can link ecosystems, which can strongly influence population, food web and community dynamics by providing organisms access to more resources and habitats (Lamberti et al. 2010; Polis et al. 1997). Understanding the trophic structure and interactions within a system can provide information on sources of productivity and nutrient pathways (Frisch et al. 2014). Species can vary in their trophic position in relation to several abiotic and biotic factors, such as seasonal food pulses, body size and choice of habitat (McMeans et al. 2019; Romanuk et al. 2011). In addition, there can be high variability in the trophic position amongst individuals of a species due to ontogeny or different behavioural responses to resource availability (Sánchez-Hernández et al. 2017; Svanbäck et al. 2015). Ontogenetic shifts in diet between size classes and life history stages are common for many fish species (Grey 2001; Power et al. 2002), where older and larger conspecifics can consume larger body-sized prey, resulting in increasing trophic position with increasing body size (Layman et al. 2005). An increase in body size, and in turn gape size, allows for the use of a broad range of resources and a greater mobility between habitats across larger spatial scales (Winemiller 1990). Therefore, quantifying ontogenetic changes in the trophic position of consumers will provide finer-scale insight into the trophic dynamics of the ecosystem.
Aquatic consumers can play an important functional role between systems due to their movements and flexible foraging behaviours (Schindler and Scheuerell 2002). Fish occupy a wide variety of trophic positions due to their morphological (gill rakers vs. teeth) and behavioural (filter feeding vs. particulate feeding) feeding patterns (Costalago et al. 2012) and play key roles in food webs. A community with high trophic diversity will result in several species occupying many different areas of the food web, resulting in more variability in habitat and resource use amongst species. In contrast, trophic redundancy occurs when multiple species function similarly in the community, resulting in these species using similar resources and occupying the same trophic position or area in the food web (Polis et al. 2000; Yurkowski et al. 2018). However, over time, the trophic structure of communities can dramatically change in relation to climate and anthropogenic effects (e.g., shipping and pollution), where the impacts of both are pronounced and rapidly increasing across the globe, but especially in the Arctic (Bartley et al. 2019; McCann et al. 2005; Pecl et al. 2017).
Changes in Arctic sea ice cover and temperatures have resulted in shifts in the distribution of many mobile consumers, leading to changes in the species composition of fish communities. Within Hudson Bay, Gaston et al. (2003) suggested decreased availability of Arctic cod to thick-billed murres (Uria lomvia) in relation to decreasing seasonal ice cover. Similar shifts in the prey base have been found throughout Hudson Bay and will continue to impact top predator marine mammals and seabirds that mainly rely on fish as a resource (Florko et al. 2021). Therefore, understanding the trophic structure of specific areas in the subarctic and Arctic, such as the lower Churchill River system in southwest Hudson Bay, is important since the degree of trophic shifts could vary throughout the Hudson Bay region.
Given the climatic changes within Hudson Bay and increasing anthropogenic activity, the lower Churchill River system in Manitoba, Canada is an ideal study site to investigate recent (2019–2020) variation in trophic position of numerous mobile consumers, allowing for a comparison of trophic diversity in the early 1990s, before the construction of the Churchill River weir. Since 1976, around 75–90% of the Churchill River flow had been impounded and diverted to hydroelectric generating stations along the Nelson River (Kuzyk et al. 2008; Newbury et al. 1984), resulting in reduced water flow along the Churchill River, which has likely affected both fresh water and estuarine communities on the river and likely fish populations. To increase water levels and improve river accessibility and fish abundance, the Churchill River weir was constructed in 1998. The weir is 13 km upstream from the river mouth and is a 3-km long rockfill dyke that extends across the river and causes water levels to rise upstream of the structure (Kuzyk et al. 2008). In-stream barriers help with transportation, water supply, flood control, agriculture and power generation. However, the barrier in the waterway may affect riverine ecosystems (Carpenter-Bundhoo et al. 2020; Poff et al. 1997). The development of weirs can interrupt the river continuum and are not only restricted to a few sensitive species or taxonomic groups but also affect the entire aquatic community structure (Mueller et al. 2011). The barrier presented by a weir can also interfere with short-term localised movements amongst habitats, which is important for foraging, escaping from predators and access to thermal refugia (Carpenter-Bundhoo et al. 2020).
The first objective of this study was to quantify the trophic positions of mobile consumers (13 fishes and 2 seal species) within the marine/freshwater food web in the lower Churchill River area, at both the individual and group levels (same species that live in a specific area that are grouped together), using stable isotope analysis (δ13C and δ15N). The second objective was to determine if there was an ontogenetic effect on trophic position of the following fish species for which we had age data: cisco, fourhorn sculpin, Greenland cod, lake whitefish and northern pike. The third objective was to quantify temporal changes in the trophic structure of a three-species fish assemblage (cisco, fourhorn sculpin and lake whitefish) between 1993–1995 and 2019–2020. These three species were used since they were the only species that were caught during both time periods and are all able to take advantage of both marine and freshwater resources. Specifically, we aimed to test the following: (1) if a species that occupies a higher trophic position will also have more variability between individuals in their trophic position, (2) if trophic position will increase with age across each studied fish species, and (3) whether the trophic diversity of a three-species fish assemblage has changed after the construction of the weir.
Materials and Methods
Study Site
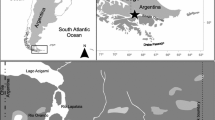
This study was conducted within the lower Churchill River area near the town of Churchill (58.7684° N, 94.1650° W), Manitoba, Canada. The Churchill River is connected to Hudson Bay through the Churchill River Estuary. Consumer and prey samples were collected during July and August of 2019 and 2020 throughout the river, the estuary and in the coastal waters of Hudson Bay. Goose Creek was the closest tributary to the estuary that was sampled (Fig. 1).
Sample Collection and Preparation
During data collection, we aimed to sample all representative fish from each habitat (marine, estuary and freshwater). As a result, thirteen fish species were collected using a combination of gillnets and angling, and then euthanized. Target fish were euthanized by a blow to the head and cervical dislocation. Varying gill net monofilament mesh sizes (2–3″, 4″ and 5″) were used within each location and a spinning reel was only used in the estuary. Each net had a height of 6′ and a length of 80′. Nets were set for a 6-h soak and were checked every 2 h to reduce bycatch of any non-target species (Manitoba Government permits: SCP 40–19 APZ and SCP 23–20 APZ). For each fish, fork length and total length (cm), mass (g) and sex were recorded, and internal structures (muscle, liver, stomach and otolith/cleithrum) were subsampled. Some northern pike (Esox lucius) samples were collected between late-May to September in 2019 and 2020, whereas all other fish samples were collected in July and August 2019 and 2020 (Supplementary Material, Table S.1). Seal samples were provided opportunistically by local hunters. Four harbour seals (Phoca vitulina) were collected in September of 2016 and five ringed seals (Pusa hispida) were collected in November 2019 and April–May 2020. During the summers of 1993–1995, fish were collected from the Churchill River and estuary using beach seines nearshore and at coastal creek mouths by gill nets (sampling details are described in Baker et al. (1993)). All samples collected were stored at − 30 °C prior to freeze-drying. Samples were processed and then run for stable isotope analysis approximately one year after they were collected.
Samples of liver and muscle, which have different turnover rates, were collected from each fish to provide an understanding of any dietary shifts over the spring–summer period (Fry 2006; Heady and Moore 2013; Phillips and Eldridge 2006). Tissues with a faster turnover rate, such as liver, provide dietary information on a weekly scale (~ 15–30 days), whereas tissues with a slower turnover rate like muscle provide information on a monthly scale (~ 1–4 months) (Vander Zanden et al. 2015). Since most samples were collected in summer (July and August), liver would represent the summer diet and muscle would represent the spring–summer diet. Smaller-sized individuals (invertebrates, three burbot (Lota lota), four nine-spine sticklebacks (Pungitius pungitius), and one sculpin (Cottoidea spp.)) had the whole body homogenised for analysis. Muscle and liver samples were collected from ringed seals, whereas muscle and hair samples were collected from harbour seals. Harbour seals moult in August (Bajzak et al. 2013; Vincent et al. 2017), so this regrown hair would represent the diet slightly before and during the moulting period due to representative elements circulating in the blood that are then deposited into the growing hair (Hobson 1999). Hair tissue is metabolically inert after growth and will maintain a stable isotope composition based on resources consumed during the hair growth period (Hobson 1999). Since pinnipeds moult their hair annually, the harbour seal samples that were collected in September will reflect their diet over a short time period (~ 1–2 months).
Tissue samples from invertebrates, fishes and seals collected from 2019 to 2020 were freeze-dried for 48 h and then homogenised by hand with a mortar and pestle. Lipids were extracted from the liver, muscle and whole-body samples with a 2:1 chloroform–methanol solution following procedures detailed in Bligh and Dyer (1959) and McMeans et al. (2009). Hair samples were washed with soap and water, rinsed thoroughly and then left to dry overnight, then homogenised by hand with scissors. Lipids were not removed from the 1990s samples; therefore, a lipid normalisation equation, using the C:N ratio, was applied to the δ13C values to correct for the potential bias of lipids in the sample. We used the equation for aquatic organisms in Post et al. (2007), δ13Cnormalized = δ13Cuntreated − 3:32 + 0:99 × C:N. This equation is accurate and commonly used with samples that have a low C:N ratio and a low range of C:N ratios (Hoffman and Sutton 2010).
Stable isotope analysis provides time-integrated information on habitat use and diet of a consumer (Fry 2006) where carbon stable isotopes (δ13C) are used to determine the source of dietary carbon (e.g., terrestrial/freshwater versus marine) and nitrogen stable isotopes (δ15N) are used to determine trophic position of consumers (Fry 2006; Heady and Moore 2013; Phillips and Eldridge 2006). For the 2019–2020 samples, stable isotope analysis was performed at the Chemical Tracers Laboratory, Great Lakes Institute for Environmental Research, at the University of Windsor during 2019–2020. A Delta V Advantage Mass spectrometer (Thermo Finnigan, San Jose, CA, USA) coupled to a Costech 4010 Elemental Combustion system (Costech, Valencia, CA, USA) and a ConFlo IV gas interface was used for the analysis. A triplicate was run for every 10th sample where precision was estimated at 0.1‰ for both δ13C and δ15N. Table S.2 in the Supplementary Material reports the percent carbon and nitrogen from the muscle and liver for each fish and seal species from the 2019–2020 samples after stable isotope analysis. The 1993–1995 samples were analysed at the Freshwater Institute in Winnipeg, Manitoba with a dual inlet isotope ratio VG Optima mass spectrometer (Isoprime Inc., Manchester, UK) attached to an elemental analyser (Carlo Erba NA1500). An external laboratory working standard (Pharmamedia, a cottonseed protein) was run every 5 to 10 samples for a measurement precision of 0.07‰ and 0.05‰ for δ13C and δ15N respectively.
Fish ages were determined at AAE Tech Services Inc. in La Salle, Manitoba. Samples were either analysed whole or sectioned (Table S.3 in the Supplementary Material). Annuli of otoliths were counted under a microscope with transmitted light. A quality assurance/quality control (QA/QC) was completed for about 60% of the 2019 samples and all the 2020 samples, where a second reader estimated the ages and then compared their age estimates to the first reader.
Data Analysis
All data analyses were conducted in R v. 4.0 (R Core Team 2021). Stable isotope values of prey and consumers can vary over time and space (Cabana and Rasmussen 1996; Fry 2006; Post 2002); therefore, to determine if the mean species-specific δ13C and δ15N values for each consumer tissue differed significantly between sampling years, a Wilcoxon signed-rank test was performed. We also used a Holm-Bonferroni sequential correction to account for multiple comparisons between sampling years (i.e., α = 0.05/(number of tests run + 1 − rank number of pair)). The δ13C and δ15N values were checked for normality with quantile–quantile plots and histograms, and for heteroscedasticity with residual-fitted value plots. Non-parametric tests were used to test for spatial and temporal similarity for all correlation tests due to having small sample sizes for some species (e.g., brook trout (Salvelinus fontinalis), burbot (Lota lota), longnose sucker (Catostomus catostomus) and white sucker (Catostomus commersonii)). Based on the Wilcoxon signed-rank test, we found no significant difference in ringed seal values between seasons; therefore, ringed seals collected from the different seasons were combined. Northern pike and lake whitefish collected in 2019 were collected during two different time periods, in May in Goose Creek and in July in the lower Churchill River area. Based on the Wilcoxon signed-rank test we found northern pike muscle and liver differed significantly between May and July for δ15N, but there was no difference for lake whitefish (Supplementary Material, Table S.4). Therefore, northern pike were separated into two groups, one from spring and a second from summer. As well, there were no significant differences between 2019 versus 2020 for δ13C and δ15N for any of the other species (Table S.5 in the Supplementary Material). The mean value for δ13C and δ15N for each species based on their sampling location can be found in Table S.6 for the 2019–2020 samples and Table S.7 for the 1993–1995 samples in the Supplementary Material.
We estimated trophic position of each consumer using the scaled trophic position approach, since diet-tissue discrimination factors (Δ15N) can vary due to protein quality and quantity, where Δ15N typically decreases with increasing prey δ15N (Hussey et al. 2014). Each species’ habitat classification was based on results from St George et al. (2023), which used the same samples to examine the percent of freshwater versus marine-derived resource use of consumers within this system via stable isotope mixing models. Based on known movement ecology and results from St George et al. (2023), we grouped together harbour seals, ringed seals, capelin, Greenland cod, fourhorn sculpin, nine-spined stickleback and brook trout as species that mainly used marine-derived resources. Northern pike (summer), burbot, white sucker, longnose sucker and trout-perch were grouped together. These were found to mainly use freshwater-derived nutrients. Cisco, lake whitefish and spring northern pike were found to be habitat couplers within this system since they made use of resources from both the freshwater and marine system (St George et al. 2023). These habitat coupling species have been known to be either anadromous (cisco and lake whitefish) or their physiology allows them to tolerate more brackish waters (northern pike). The movement ecology and habitat use for the species that consumed freshwater-derived resources and marine-derived resources in our study were consistent with their known life histories. Therefore, trophic position for each mobile consumer was calculated using a one-source trophic position model (Post 2002):
where the δ15N of freshwater mussels (Unionida) (4.67 ± 0.03‰) was used as a baseline (δ15Nbaseline) to estimate the trophic position for freshwater fishes, and the δ15N of blue mussels (Mytilus edulis) (8.58 ± 0.15‰) was used to estimate the trophic position for marine fishes. Bivalvia consume microalgae, organic matter and detritus falling from the water’s surface and therefore feed at trophic position 2, which is assigned as the baseline value (TPbaseline) for the fishes (Jung et al. 2019; McMeans et al. 2013). We used freshwater mussels and blue mussels as the baseline prey species since δ15N values can vary between marine and freshwater systems (Hesslein et al. 1991); therefore, using prey based on the known diet of the mobile consumers can account for that variation in δ15N between each connected system (δ15Nconsumer). Most species principally consumed either freshwater-derived resources or marine-derived resource. Only three species were found to have a mix of both freshwater and marine-derived resources. For harbour seals and ringed seals, we used the δ15N of capelin (14.71 ± 0.09‰) as the baseline, with the TPbaseline = 3.69, since both predators are mainly piscivorous and consume capelin (Ogloff et al. 2020; Thiemann et al. 2008). For the δ15N of fishes, a diet-tissue discrimination factor of 3.67‰ was used for muscle and 2.80‰ was used for liver (Caut et al. 2009; Hobson et al. 1996; McCutchan et al. 2003). For ringed seals and harbour seals, we used 2.40‰, 3.10‰ and 3.00‰ for muscle, liver and hair, respectively (Caut et al. 2009; Hobson et al. 1996; McCutchan et al. 2003).
Kendall’s tau correlation tests were run to examine the correlation between a species’ trophic position and the range of trophic positions amongst individuals. We also examined the age and body length relationships with trophic position using linear regression for fish species with a sample size > 10. For the correlation and linear regression, significance was set to an α of 0.05. A Bayesian inference package, SIBER v 2.1.6, in R was used to quantify the trophic diversity of the three-species fish assemblage (cisco, fourhorn sculpin (Myoxocephalus quadricornis) and lake whitefish) in the lower Churchill River system using six community-wide metrics (Jackson et al. 2011). Both cisco and lake whitefish are considered anadromous species and therefore are ideal representatives of species that can use both aquatic systems; fourhorn sculpin are considered a marine and estuarine species and therefore may consume both the marine and freshwater resources found within the estuary. Fish were collected from 1993–1995 before weir construction in 1998 and then approximately two decades afterwards (2019–2020). We corrected for the Suess effect since there was a 25-year gap between the sampling periods. The Suess effect represents a decline in the δ13C values of dissolved inorganic carbon due to the increased atmospheric CO2 since the industrial period, which is resulting in an increase in oceanic CO2 (Quay et al. 2003). de la Vega et al. (2019) reported a change of − 0.01‰ per year in δ13C values of dissolved inorganic carbon in the Arctic. Therefore, we subtracted 0.25‰ from the 1993–1995 data.
For each time period, we used SIBER to calculate four metrics measuring trophic diversity (δ13C range, δ15N range, mean distance to centroid and total community area) and two metrics that estimate the extent of trophic redundancy (mean distance of the nearest neighbour and standard deviation of the nearest neighbour) (Jackson et al. 2011; Layman et al. 2007). The standard ellipses represent 40% of the data points within the ellipse to infer the group’s core niche size. The convex hull represents the total area of each community, and is a polygon that is drawn around the outermost points in such that all other points lie within the outline (Jackson et al. 2011). Estimates for the overlap metrics between the 40% ellipses generated for the three examined fish species (cisco, fourhorn sculpin and lake whitefish). The δ13C and δ15N ranges are the distances between the fish assemblage in the community with the highest and lowest δ13C and δ15N values. These ranges represent the variability in basal carbon sources and relative trophic position between both time periods. Total community area is the total area between the means of each species’ isotopic niche and is less biassed by convex hull extremities. The mean distance to centroid is the average Euclidean distance of each species to the δ13C-δ15N centroid of the entire community and therefore represents species spacing between one another and provides information on the overall degree of trophic diversity. Mean nearest neighbour distance is the mean of the Euclidean distances to each species’ nearest neighbour in bi-plot space and represents the density of species packing. The standard deviation of the nearest neighbour is a measure of the evenness of species packing in bi-plot space and is less influenced by sample size than the nearest neighbour distance (Jackson et al. 2011; Layman et al. 2007; Yurkowski et al. 2018). All metrics were derived from 2,000,000 iterations, with a burn-in of 100,000, and thinned by 10, leaving 90,000 posterior estimates from the posterior probability distribution.
Results
Stable Isotopes
Consumer species with the lowest average δ13C values were walleye (Sander vitreus) (-28.34‰), whereas the species with the highest average δ13C values were ringed seals (-19.85 ± 0.33‰). Spring northern pike had the lowest average δ15N values (6.78 ± 0.22‰), whereas harbour seals had the highest δ15N value (18.06 ± 0.19‰) (Table 1, Table S.8 in the Supplementary Material for the liver results). Three groups were characterised by the δ13C and δ15N muscle biplots (Fig. 2): (1) consumers with higher δ13C and δ15N values, which included harbour seal, ringed seal, brook trout, capelin, cisco, fourhorn sculpin, Greenland cod (Gadus ogac) and nine-spine stickleback, (2) fish with the lowest δ13C and δ15N values, which included burbot, longnose sucker, summer northern pike, spring northern pike, trout-perch (Percopsis omiscomaycus), walleye and white sucker and (3) lake whitefish, which had intermediate δ13C and δ15N values. Similar grouping of species also occurred with liver tissue, although more species were included in the intermediate group, such as burbot, lake whitefish, summer northern pike and spring northern pike. Results from muscle and liver were nearly identical for most of the species; therefore, results are interpreted from muscle in this study, with results from liver tissue provided in the Supplementary Material. However, results from liver tissue are reported when different from muscle.
Stable isotope biplot of the mean (‰) ± standard error (SE) of δ13C and δ15N of the 15 consumers within the Churchill River and Churchill River estuary based on their different tissue type and two prey used as the prey baseline for the trophic position equation. Hair samples were used for the harbour seals instead of liver
Trophic Positions
The trophic positions of consumers were rounded to the nearest trophic level and classified into groups. For example, secondary consumer species that occupied a trophic level of 3 could range between a trophic position between 2.5 and 3.4 (Woodland et al. 2016). During the spring–summer period, the secondary consumers (trophic position ~ 3) in this system were spring northern pike, longnose sucker, white sucker, lake whitefish, burbot, summer northern pike, cisco and trout-perch. Greenland cod, brook trout, capelin, fourhorn sculpin, nine-spine stickleback and walleye occupied the tertiary consumer position (trophic position ~ 4) and harbour and ringed seals were the apex predators (trophic position ~ 5; Table 1 and Fig. 3). Overall, the trophic positions for most species were similar between muscle and liver tissue. However, cisco, burbot and summer northern pike had a slightly higher trophic position in summer (reflected in liver) than the spring–summer time period (reflected in muscle) (Figure S.1 in the Supplementary Material).
The highest level of variation in trophic position between individuals occurred in lake whitefish (muscle: 2.07–4.19, n = 68) and nine-spine stickleback (muscle: 1.54–4.37, n = 5), and the lowest level of variation between individuals was observed in trout-perch (muscle: 3.27–3.52, n = 19) (Table 1). Individual variability did not increase with trophic position (muscle: z = 0.20, p = 0.84, liver: z = − 0.43, p = 0.67). Fish age was positively correlated with body size (z = 11.10, p < 0.001, tau = 0.59). Trophic position increased with age in cisco (β = 0.042, p = 0.004, R2 = 0.29), fourhorn sculpin (β = 0.063, p = 0.04, R2 = 0.16), Greenland cod (β = 0.21, p < 0.001, R2 = 0.59) and summer northern pike (β = 0.055, p < 0.001, R2 = 0.69; Fig. 4). We also found trophic position increased with body size for lake whitefish (muscle: β = 0.001, p = 0.03, R2 = 0.07). See Table S.9 in the Supplementary Material for morphometric measurements of each species.
The relationship between age and trophic position of six fish groups (cisco, fourhorn sculpin, Greenland cod, lake whitefish, summer northern pike and spring northern pike) within the Churchill River and Churchill River estuary area based on a muscle and b liver. *Species with a significant relationship between body size and trophic position
Community-Wide Metrics
The sizes of the Bayesian standard ellipses (SEAb) and corrected standard ellipses areas (SEAc) were larger in 2019–2020 than in 1993–1995 for cisco (SEAb: recent = 2.14‰2, past = 1.97‰2; SEAc: recent = 2.28‰2, past = 2.07‰2) and fourhorn sculpin (SEAb: recent = 2.30‰2, past = 0.96‰2; SEAc: recent = 2.47‰2, past = 1.16‰2), whereas the SEAb and SEAc was smaller for lake whitefish (SEAb: recent = 9.26‰2, past = 16.00‰2; SEAc: recent = 9.31‰2, past = 17.52‰2). Niche overlap was highest between cisco and fourhorn sculpin for both time periods, but slightly higher in 1993–1995 (0.27%) than in 2019–2020 (0.12%). As well, the overlap percentage was lower in the 2019–2020 community than the 1993–1995 community. All six of the community-wide metrics were lower in 2019–2020 than in 1993–1995 (Figs. 5 and 6). From the posterior distribution, the probability that total area was smaller in 2019–2020 than in 1993–1995 was 67%, which was likely a result of an increase in mean δ13C for lake whitefish combined with a decrease in mean δ13C for both cisco and fourhorn sculpin (Fig. 5). The probability that δ13C range (variability in basal carbon source) and δ15N range (range of trophic positions) were lower in 2019–2020 versus 1993–1995 was 100% and 91%, respectively. A decrease in trophic diversity also occurred, as the probability that mean distance to centroid was lower in the more recent time period was 100%. As such, trophic redundancy increased, as the probability of the mean nearest neighbour distance and standard deviation of nearest neighbour distance was lower in 2019–2020 than in 1993–1995 was 76% and 99%, respectively.
Stable isotope biplot representing the 40% isotope niche sizes (ellipses) of cisco, fourhorn sculpin and lake whitefish during 1993–1995 and 2019–2020 within the Churchill River and Churchill River estuary. The dashed lines represent the community metric of total area. The solid ellipses lines and closed circles are for the 1993–1995 community and the dashed ellipses lines and open circles are for the 2019–2020 community
Boxplots representing the Bayesian estimates for each community-wide metric for the 3-species fish assemblage from within the Churchill River and Churchill River estuary including (a) δ13C range ‰, (b) δ15N range ‰, (c) mean distance to centroid ‰, (d) total area ‰2, (e) mean nearest neighbour distance ‰, and (f) standard deviation of nearest neighbour distance ‰. The dot in the centre of the boxes represents the mode. Boxes indicate Bayesian credible intervals at 50% (dark grey), 75% (medium grey) and 95% (light grey)
Discussion
Trophic Position
Trophic positions of fishes and marine mammals ranged from 2.57 to 5.09 in the Lower Churchill River system, which is comparable to other aquatic systems (Roach et al. 2009; Power et al. 2002). Secondary consumers (trophic position ~ 3) in the lower Churchill River system included spring northern pike, longnose sucker, white suckers, lake whitefish, burbot, summer northern pike, cisco and trout-perch, and nine-spine stickleback. Northern pike are generalist consumers that feed on a variety of resources from invertebrates to fish (Harvey 2009). Northern pike (mean trophic position 3.38) sampled in summer in the Churchill River occupied a higher trophic position than northern pike (mean trophic position 2.57) sampled in spring from Goose Creek, suggesting a gradual ontogenetic shift to larger prey (Graeb et al. 2006). Summer northern pike collected in the river were larger than the spring northern pike collected in Goose Creek, but it is unknown if this diet change is due to age/size effects or if it could possibly be from changes in the prey availability during the different seasons.
Longnose sucker (mean trophic position 2.86) and white sucker (mean trophic position 2.96) are known to forage on algae and benthic invertebrates (Edwards 1983; Saint-Jacques et al. 2000) and there was little variation amongst individuals in both species. Lake whitefish (mean trophic position 3.05), northern pike and cisco (mean trophic position 3.40) are known to have an anadromous life history and can tolerate brackish waters (Morin et al. 1981; Rohtla et al. 2012; Wilder 1951), which can allow them access to resources from both the marine and freshwater systems. Cisco, northern pike (spring) and lake whitefish were found to be habitat-coupling species within our study (St George et al. 2023). Lake whitefish forage on benthic prey, such as amphipods, snails and mussels (Rennie et al. 2009). The greatest variation in trophic position between individuals occurred in lake whitefish, ranging from primary consumers to tertiary consumers, which is likely due to their different foraging strategies as a species. Some individuals of lake whitefish are potentially focusing on invertebrates whilst others are foraging principally on mid-trophic level fish. Variability was also found between individuals where some primarily consume higher trophic position marine resources, whilst other individuals mainly consume lower trophic position freshwater resources. Cisco are known to be planktivorous, and feed mainly on cladocerans and copepods (Viljanen 1983). Cisco were also found to be habitat couplers within the lower Churchill River and foraged on more marine-derived resources (St George et al. 2023), which also may explain their higher trophic position amongst secondary consumer species. Burbot (mean trophic position 2.90) are omnivorous and consume insects, amphipods and other fish (Beeton 1956; Lawler 1963). Trout-perch (mean trophic position 3.40) are generalists that forage on benthic invertebrates and zooplankton, especially chironomids and mayflies, as well as some fish eggs and smaller fish (Blouzdis et al. 2013; Kocovsky et al. 2014; Nelson and Dick 2002). Nine-spine sticklebacks (mean trophic position 3.36) are also generalists and will forage on small crustaceans, aquatic insects and eggs and fry of fish (Hynes 1950).
Tertiary consumers (trophic position of ~ 4) were walleye, capelin, brook trout, fourhorn sculpin and Greenland cod. Top predators (trophic position 5) were harbour seals and ringed seals. Tertiary consumers can be omnivorous by foraging on a variety of prey items from different invertebrates and fish species that occupy a mix of mid-level trophic positions (Blouzdis et al. 2013; Galarowicz et al. 2006; Lawler 1963; Morin and Dodson 1986; Vesin et al. 1981). However, the top consumers in this study had small niche sizes, showing that they were mainly using one resource type as observed in St George et al. (2023). Walleye (mean trophic position 3.64 from Table 1) are known to forage on zooplankton and fish when they are juveniles (~ 20 mm), but at larger body sizes (40–100 mm) switched to benthic invertebrates and fish (Galarowicz 2006). Capelin (mean trophic position 3.67) feed mainly on zooplankton, such as amphipods, copepods and euphausiids (Ogloff et al. 2020; Vesin et al. 1981). Brook trout (mean trophic position 3.81) have an anadromous life history stage and usually migrate into marine systems in spring (Black et al. 1983). Brook trout forage on species available in the water current and benthos (Fechney 1988) and feed on minnows, sticklebacks, perch and sculpins (Ricker 1930).
Fourhorn sculpin (mean trophic position 3.77) are a benthic fish that consume mainly amphipods; however, they can also forage on plant material, molluscs, insects, mysids, polychaetes and fish (Morin and Dodson 1986). Greenland cod (mean trophic position 4.29) are considered a top predator within the shallow benthic food chains of Hudson Bay and consume fish such as capelin (Mikhail and Welch 1989; Mouritsen et al. 2010). Ringed seals (mean trophic position 4.70) and harbour seals (mean trophic position 5.09) mainly consume fishes, such as capelin and Arctic cod, and therefore occupy the top trophic position within this system (Ogloff et al. 2020; Thiemann et al. 2008; Yurkowski et al. 2016). Harbour seals are central place foragers and typically forage in marine waters, but in this region, harbour seals haul out on rocks in the Churchill River estuary and some individuals also move upriver, presumably to forage on freshwater-derived resources (Bajzak et al. 2013).
We observed a pattern where species that primarily consumed more marine-derived resources occupied higher trophic positions than species that mainly consumed more freshwater-derived resources. Marine ecosystems are generally considered to be more productive than freshwater systems and consumer omnivory is usually found more often in marine systems compared to freshwater systems (Sánchez-Hernández and Amundsen 2018). Sánchez-Hernández and Amundsen (2018) used data from FishBase and compared trophic position and omnivory of fishes amongst different ecosystem types. They found that trophic position increased from freshwater to marine species for filter feeding, zoobenthos, benthopelagic, demersal, tropical, subtropical and temperate species but that this relationship did not occur in herbivorous species due to their obligate consumption of primary producers. The shifts from low to high trophic positions between aquatic systems is likely due to spatial differences in prey availability, where marine systems are typically more diverse than freshwater systems at the same latitude (Sánchez-Hernández and Amundsen 2018).
Body Size-Trophic Position Relationship
Size-related constrains on prey consumption (i.e., gape size) can result in a mechanical limit to the trophic position of consumers (Scharf et al. 2000) where larger consumers typically consume smaller-sized prey items (Keppeler et al. 2021). The trophic position of cisco, fourhorn sculpin and Greenland cod, species that mainly consume marine-derived resources, and summer northern pike, which mainly consume freshwater-derived resources, increased with age. Positive trophic position-body size relationships occur in both marine and freshwater systems, but are more common in marine systems than in freshwater and terrestrial systems (Potapov et al. 2019; Keppeler et al. 2021). Cisco are known to change their diet with increasing body size, with a shift towards larger zooplankton that would increase their trophic position. Smaller individuals of sculpin and Greenland cod typically forage on invertebrates, whilst larger individuals forage on fishes (Dalponti et al. 2018; Landry et al. 2018), which may also occur in fourhorn sculpin. Beaudoin et al. (1999) found a positive relationship between δ15N and body size for pike and suggested that northern pike may have more trophic flexibility, which can vary in the presence or absence of other fish species.
Shifts in Community Structure
The decrease in δ13C of cisco and fourhorn sculpin between 1993–1995 and 2019–2020 could be due to an increased consumption of freshwater/terrestrial-derived resources over time or a switch to more pelagic prey, whereas the increase in the δ13C of lake whitefish may be a result of increased consumption of marine-derived resources or a switch to more benthic food sources in the more recent time period. Water barriers, such as weirs, cause may cause physical barriers that could affect the ability for fish to move between different habitats to complete their life-history requirements (Carpenter-Bundhoo et al. 2020). Weirs that periodically drown out can alter aquatic habitat by changing river hydraulics, which could affect downstream habitats and resource availability (de Leaniz et al. 2019; Tiemann et al. 2004). As well, within large rivers, seasonal variation in discharge rates may affect population dynamics by influencing prey growth rates, predator–prey interactions and availability of refuge habitats (Power et al. 1995). Therefore, discharge patterns could also be influencing the baseline prey, which could be contributing to changes observed in the community structure. Cisco, fourhorn sculpin and lake whitefish are known to mainly forage on invertebrates, but these species can also forage on several different fish species (Morin and Dodson 1986; Rennie et al. 2009; Viljanen 1983). Cisco and lake whitefish couple the marine and freshwater systems together (DeJong 2017; St George 2023), which illustrates their high variability in resource use in consuming a variety of freshwater and marine invertebrates and fishes (Morin and Dodson 1986; Rennie et al. 2009; Viljanen 1983). For all three fish species, δ15N increased over time, suggesting an increase in the trophic position of each consumer that may be due to changes in the prey composition and abundance within the Churchill River and estuary. For example, grayling (Thymallus arcticus), a lower trophic level consumer that exhibits a flexible foraging strategy by consuming amphipods, insects and fish (Stewart et al. 2007), has likely been extirpated from the area that coincided with warming water temperatures and hydroelectric activity (Edye-Rowntree 2007). Grayling typically occupy a similar niche to lake whitefish and cisco (Laske et al. 2018); therefore, after the loss of grayling in this system, there may have been increased prey availability for both lake whitefish and cisco.
Overall, the increase in trophic redundancy within the 2019–2020 community compared to the early 1990s suggests that all three fish species are now playing a more similar trophic role within the food web than they did before. Increased trophic diversity can promote ecosystem stability since there are many species occupying distinct trophic positions across the system, where different species partition and consume different food items (Włodarska-Kowalczuk et al. 2019). When ecosystem stability is reduced it may foreshadow more species loss within this system due to competition and/or if there is a reduction in their prey source (Bartley et al. 2019; Magoulick and Piercey 2015; Yurkowski et al. 2017). However, in some cases, greater trophic redundancy may help promote ecosystem stability and reduce vulnerability to secondary extinction events (Sanders et al. 2018) where the loss of one species in the system is replaced by another species with the same functional role. As such, it is unknown whether the change in both trophic diversity and trophic redundancy in the three fish species assemblage has increased or decreased ecosystem stability in the lower Churchill River system, which requires further investigation.
Conclusion
This study is the first to characterise the trophic structure of the lower Churchill River system. Trophic position estimates varied widely (1.39–5.21 for muscle) across consumers. Species that consumed more marine-derived resources occupied higher trophic positions than species that principally consumed freshwater-derived resources. The results from this study can be used to compare with future research, thereby providing a time series of monitoring changes in the trophic position of these consumers species throughout the lower Churchill River. The community structure of the three-fish species assemblage within the Churchill River and estuary currently has lower trophic diversity and higher trophic redundancy compared to the early 1990s. Both climate change and hydroelectric development likely resulted in temporal changes to the available habitat and resources within the system, thereby influencing the trophic structure, which has implications for the management and conservation of subarctic and Arctic ecosystems.
Data Availability
Data will be made available on request.
References
Bajzak, C.E., W. Bernhardt, A. Mosnier, M.O. Hammill, and I. Stirling. 2013. Habitat use by harbour seals (Phoca vitulina) in a seasonally ice-covered region, the western Hudson Bay. Polar Biology 36 (4): 477–491. https://doi.org/10.1007/s00300-012-1274-4.
Baker, R.F., M.J. Lawrence, and F. Schneider. 1993. Oceanography and mid-summer distribution and abundance of plankton and fish in the Nelson Estuary. Winnipeg, Canada: Hudson Bay. Report prepared for Manitoba Hydro. North/South Consultants Inc.
Bartley, T.J., K.S. McCann, C. Bieg, K. Cazelles, M. Granados, M.M. Guzzo, A.S. MacDougall, T.D. Tunney, and B.C. McMeans. 2019. Food web rewiring in a changing world. Nature Ecology & Evolution 3 (3): 345–354. https://doi.org/10.1038/s41559-018-0772-3.
Beaudoin, C.P., W.M. Tonn, E.E. Prepas, and L.I. Wassenaar. 1999. Individual specialization and trophic adaptability of northern pike (Esox lucius): An isotope and dietary analysis. Oecologia 120 (3): 386–396. https://doi.org/10.1007/s004420050871.
Beeton, A.M. 1956. Food habits of the burbot (Lota lota lacustris) in the White River, a Michigan trout stream. Copeia 1956 (1): 58–60.
Black, G.A., W.L. Montgomery, and F.G. Whoriskey. 1983. Abundance and distribution of Salmincola edwardsii (Copepoda) on anadromous brook trout Salvelinus fontinalis (Mitchill) in the Moisie River system Quebec. Journal of Fish Biology 22 (5): 567–575. https://doi.org/10.1111/j.1095-8649.1983.tb04216.x.
Bligh, E.G., and W.J. Dyer. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37 (8): 911–917. https://doi.org/10.1139/y59-099.
Blouzdis, C.E., L.N. Ivan, S.A. Pothoven, C.R. Rosewell, C.J. Folet, and T.O. Höök. 2013. A trophic bottleneck?: The ecological role of trout-perch Percopsis omiscomaycus in Saginaw Bay, Lake Huron. Journal of Applied Ichthyology 29 (2): 416–424. https://doi.org/10.1111/jai.12023.
Cabana, G., and J.B. Rasmussen. 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Science 93 (20): 10844–10847. https://doi.org/10.1073/pnas.93.10844.
Carpenter-Bundhoo, L., G.L. Butler, N.R. Bond, S.E. Bunn, I.V. Reinfelds, and M.J. Kennard. 2020. Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species. Scientific Reports 10 (1): 6817. https://doi.org/10.1038/s41598-020-63005-8.
Caut, S., E. Angulo, and F. Courchamp. 2009. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. The Journal of Applied Ecology 46 (2): 443–453. https://doi.org/10.1111/j.1365-2664.2009.01620.x.
Costalago, D., J. Navarro, I. Álvarez-Calleja, and I. Palomera. 2012. Ontogenetic and seasonal changes in the feeding habits and trophic level of two small pelagic fish species. Marine Ecology 460: 169–181. https://doi.org/10.3354/meps09751.
Dalponti, G., R.D. Guariento, and A. Caliman. 2018. Hunting high or low: Body size drives trophic position among and within marine predators. Marine Ecology Process Series 597: 39–46. https://doi.org/10.3354/meps12584.
de la Vega, C., R.M. Jeffreys, R. Tuerena, R. Ganeshram, and C. Mahaffey. 2019. Temporal and spatial trends in marine carbon isotopes in the Arctic Ocean and implications for food web studies. Global Change Biology 25 (12): 4116–4130. https://doi.org/10.1111/gcb.14832.
de Leaniz, C.G., A. Berkhuysen, and B. Belletti. 2019. Deware small dams, they can do damage, too. Nature 570 (7760): 164. https://doi.org/10.1038/d41586-019-01826-y.
DeJong, R.A. 2017. Life history characteristics of lake whitefish (Coregonus clupeaformis), cisco (Coregonus artedi), and northern pike (Esox lucius) in rivers of the Hudson Bay Lowlands. (Masters thesis). University of Waterloo, Ontario, Canada.
Edwards, E.A. (1983). Habitat suitability index models. Longnose sucker. Washington, DC: Western Energy and Land Use Team, Division of Biological Services, Research and Development, Fish and Wildlife Service, U.S. Dept. of the Interior.
Edye-Rowntree, J. 2007. Churchill residents’ use of the lower Churchill River in Manitoba. (Masters thesis) University of Manitoba, Winnipeg, Canada.
Fechney, L.R. 1988. The summer diet of brook trout (Salvelinus fontinalis) in a South Island high-country stream. New Zealand Journal of Marine and Freshwater Research 22 (2): 163–168. https://doi.org/10.1080/00288330.1988.9516288.
Florko, K.R.N., T.C. Tai, W.W.L. Cheung, S.H. Ferguson, U.R. Sumaila, D.J. Yurkowski, M. Auger-Méthé, and N. Bruyn. 2021. Predicting how climate change threatens the prey base of Arctic marine predators. Ecology Letters 24 (12): 2563–2575. https://doi.org/10.1111/ele.13866.
Frisch, A.J., M. Ireland, and R. Baker. 2014. Trophic ecology of large predatory reef fishes: Energy pathways, trophic level, and implications for fisheries in a changing climate. Marine Biology 161 (1): 61–73. https://doi.org/10.1007/s00227-013-2315-4.
Fry, B. 2006. Stable isotope ecology. New York, NY: Springer. https://doi.org/10.1007/0-387-33745-8.
Galarowicz, T.L., J.A. Adams, and D.H. Wahl. 2006. The influence of prey availability on ontogenetic diet shifts of a juvenile piscivore. Canadian Journal of Fisheries and Aquatic Sciences 63 (8): 1722–1733. https://doi.org/10.1139/f06-073.
Gaston, A.J., K. Woo, and J.M. Hipfner. 2003. Trends in forage fish populations in northern Hudson Bay since 1981, as determined from the diet of nestling thick-billed murres Uria Iomvia. Arctic 56 (3): 227–233. https://doi.org/10.14430/arctic618.
Graeb, B.D.S., M.T. Mangan, J.C. Jolley, D.H. Wahl, and J.M. Dettmers. 2006. Ontogenetic changes in prey preference and foraging ability of yellow perch: Insights based on relative energetic return of prey. Transactions of the American Fisheries Society 135 (6): 1493–1498. https://doi.org/10.1577/T05-063.1.
Grey, J. 2001. Ontogeny and dietary specialization in brown trout (Salmo trutta L.) from Loch Ness, Scotland, examined using stable isotopes of carbon and nitrogen. Ecology of Freshwater Fish 10(3): 168–176. https://doi.org/10.1034/j.1600-0633.2001.100306.x.
Harvey, B. 2009. A biological synopsis of northern pike (Esox lucius). Canadian Manuscript Report of Fisheries and Aquatic Sciences 2885(31).
Heady, W., and J. Moore. 2013. Tissues turnover and stable isotope clocks to quantify recourse shifts in anadromous rainbow trout. Oecologia 172 (1): 21–34. https://doi.org/10.1007/s00442-012-2483-9.
Hesslein, R.H., M.J. Capel, D.E. Fox, and K.A. Hallard. 1991. Stable isotopes of sulfur, carbon, and nitrogen as indicators of trophic level and fish migration In the Lower Mackenzie River basin. Canadian Journal of Fisheries and Aquatic Sciences 48: 2258–2265.
Hobson, K.A. 1999. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 120 (3): 314–326. https://doi.org/10.1007/s004420050865.
Hobson, K.A., D.M. Schell, D. Renouf, and E. Noseworthy. 1996. Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: Implications for dietary reconstructions involving marine mammals. Canadian Journal of Fisheries and Aquatic Sciences 53 (3): 528–533.
Hoffman, J.C., and T.T. Sutton. 2010. Lipid correction for carbon stable isotope analysis of deep-sea fishes. Deep-Sea Research Part I: Oceanographic Research Papers 57 (8): 956–964. https://doi.org/10.1016/j.drs.2010.05.003.
Hussey, N.E., M.A. MacNeil, B.C. McMeans, J.A. Olin, S.F.J. Dudley, G. Cliff, S.P. Wintner, S.T. Fennessy, A.T. Fisk, and D. Hessen. 2014. Rescaling the trophic structure of marine food webs. Ecology Letters 17 (2): 239–250. https://doi.org/10.1111/ele.12226.
Hynes, H.B.N. 1950. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. The Journal of Animal Ecology 19 (1): 36–58. https://doi.org/10.2307/1570.
Jackson, A.L., R. Inger, A.C. Parnell, and S. Bearhop. 2011. Comparing isotopic niche widths among and within communities: SIBER-stale isotope Bayesian ellipse in R. The Journal of Animal Ecology 80 (3): 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x.
Jung, A.S., H.W. van der Veer, M.T.J. van der Meer, C.J.M. Philippart, and C.F. Rodrigues. 2019. Seasonal variation in the diet of estuarine bivalves. PLoS ONE 14 (6): e0217003. https://doi.org/10.1371/journal.pone.0217003.
Keppeler, F.W., J.A. Olin, P.C. López-Duarte, M.J. Polito, L.M. Hooper-Bùi, S.S. Taylor, N.N. Rabalais, F.J. Fodrie, B.J. Roberts, R.E. Turner, C.W. Martin, and O.P. Jensen. 2021. Body size, trophic position, and the coupling of different energy pathways across a saltmarsh landscape. Limnology and Oceanography Letters 6 (6): 360–368. https://doi.org/10.1002/lol2.10212.
Kocovsky, P.M., A.T. Stoneman, and R.T. Kraus. 2014. Ecology and population status of trout-perch (Percopsis omiscomaycus) in western Lake Erie. Journal of Great Lakes Research 40 (1): 208–214. https://doi.org/10.1016/j.jglr.2013.09.004.
Kuzyk, Z., R. Macdonald, M. Granskog, R. Scharien, R. Galley, C. Michel, D. Barber, and G. Stern. 2008. Sea ice, hydrological, and biological processes in the Churchill River estuary region, Hudson Bay. Estuarine, Coastal and Shelf Science 77 (3): 369–384. https://doi.org/10.1016/j.ecss.2007.09.030.
Lamberti, G.A., D.T. Chaloner, and A.E. Hershey. 2010. Linkages among aquatic ecosystems. Journal of the North American Benthological Society 29 (1): 245–263. https://doi.org/10.1899/08-166.1.
Landry, J.J., A.T. Fish, D.J. Yurkowski, N.E. Hussey, T. Dick, R.E. Crawford, and S.T. Kessel. 2018. Feeding ecology of a common benthic fish, shorthorn sculpin (Myoxocephalus scorpius) in the high arctic. Polar Biology 41 (10): 2091–2102. https://doi.org/10.1007/s00300-018-2348-8.
Laske, S.M., A.E. Rosenberger, M.S. Wipfli, and C.E. Zimmerman. 2018. Generalist feeding strategies in Arctic freshwater fish: A mechanism for dealing with extreme environments. Ecology of Freshwater Fish 27 (3): 767–784. https://doi.org/10.1111/eff.12391.
Lawler, G.H. 1963. The biology and taxonomy of the Burbot, Lota lota, in Heming Lake, Manitoba. Journal of the Fisheries Research Board of Canada 20 (2): 417–433. https://doi.org/10.1139/f63-033.
Layman, C.A., D.A. Arrington, C.G. Montana, and D.M. Post. 2007. Can stable isotope ratios provide for community-wide measurements of trophic structure? Ecology 88 (1): 42–48. https://doi.org/10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO2.
Layman, C.A., K.O. Winemiller, D.A. Arrington, and D.B. Jepsen. 2005. Body size and trophic position in a diverse tropical food web. Ecology 86 (9): 2530–2535. https://doi.org/10.1890/04-1098.
Magoulick, D.D., and G.L. Piercey. 2015. Trophic overlap between native and invasive stream crayfish. Hydrobiologia 766 (1): 237–246. https://doi.org/10.1007/s10750-015-2457-0.
McCann, K.S., J.B. Rasmussen, and J. Umbanhowar. 2005. The dynamics of spatially coupled food webs. Ecology Letters 8 (5): 513–523. https://doi.org/10.1111/j.1461-0248.2005.00742.x.
McCutchan, J.H., W.M. Lewis, C. Kendall, and C.C. McGrath. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102 (2): 378–390. https://doi.org/10.1034/j.1600-0706.2003.12098.x.
McMeans, B.C., T. Kadoya, T.K. Pool, G.W. Holtgrieve, S. Lek, H. Kong, K. Winemiller, V. Elliott, N. Rooney, P. Laffaille, and K.S. McCann. 2019. Consumer trophic positions respond variably to seasonally fluctuating environments. Ecology 100(2): e02570-n/a. https://doi.org/10.1002/ecy.2570.
McMeans, B.C., J.A. Olin, and G.W. Benz. 2009. Stable-isotope comparisons between embryos and mothers of a placentatrophic shark species. Journal of Fish Biology 75 (10): 2464–2474. https://doi.org/10.1111/j.1095-8649.2009.02402.x.
McMeans, B.C.N., M.T. Arts. Rooney, and A.T. Fisk. 2013. Food web structure of a coastal Arctic marine ecosystem and implications for stability. Marine Ecology 482: 17–28. https://doi.org/10.3354/meps10278.
Mikhail, M.Y., and H.E. Welch. 1989. Biology of Greenland cod, Gadus ogac, at Saqvaqjuac, northwest coast of Hudson Bay. Environmental Biology of Fishes 26 (1): 49–62. https://doi.org/10.1007/BF00002475.
Morin, R., J.J. Dodson, and G. Power. 1981. The migrations of anadromous cisco (Coregonus artedii) and lake whitefish (C. clupeaformis) in estuaries of eastern James Bay. Canadian Journal of Zoology 59(8): 1600–1607. https://doi.org/10.1139/z81-219.
Morin, R. and J.J. Dodson. 1986. Chapter 15 the ecology of fishes in James Bay, Hudson Bay and Hudson Strait. Elsevier Oceanography Series 44: 293–326. https://doi.org/10.1016/S0422-9894(08)70908-5.
Mouritsen, K.N., R. Hedeholm, H.B. Schack, L.N. Møller, M. Storr-Paulsen, J. Dzido, and J. Rokicki. 2010. Occurrence of anisakid nematodes in Atlantic cod (Gadus morhua) and Greenland cod (Gadus ogac). West Greenland. Acta Parasitologica 55 (1): 81–89. https://doi.org/10.2478/s11686-010-0009-3.
Mueller, M., J. Pander, and J. Geist. 2011. The effects of weirs on structural stream habitat and biological communities: Effects of weirs on streams. The Journal of Applied Ecology 48 (6): 1450–1461. https://doi.org/10.1111/j.1365-2664.2011.02035.x.
Nelson, P.A., and T.A. Dick. 2002. Factors shaping the parasite communities of trout-perch, Percopsis omiscomaycus Walbaum (Osteichthyes: Percopsidae), and the importance of scale. Canadian Journal of Zoology 80 (11): 1986–1999. https://doi.org/10.1139/z02-188.
Newbury, R.W., G.K. McCullough, and R.E. Hecky. 1984. The Southern Indian Lake impoundment and Churchill River diversion. Canadian Journal of Fisheries and Aquatic Sciences 41 (4): 548–557. https://doi.org/10.1139/f84-068.
Ogloff, W.R., S.H. Ferguson, R.F. Tallman, and G.K. Davoren. 2020. Diet of capelin (Mallotus villosus) in the Eastern Canadian Arctic inferred from stomach contents and stable isotopes. Polar Biology 43 (9): 1273–1285. https://doi.org/10.1007/s00300-020-02707-1.
Pecl, G.T., M.B. Araujo, J. Bell, J. Blanchard, T.C. Bonebrake, I. Chen, T.D. Clark, R.K. Colwell, F. Danielsen, B. Evengard, and S. Robinson. 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355 (6332): 1–9.
Phillips, D., and P. Eldridge. 2006. Estimating the timing of diet shifts using stable isotopes. Oecologia 147 (2): 195–203. https://doi.org/10.1007/s00442-005-0292-0.
Poff, N.L., J.D. Allan, M.B. Bain, J.R. Karr, K.L. Prestegaard, B.D. Richter, R.E. Sparks, and J.C. Stromberg. 1997. The natural flow regime. BioScience 47 (11): 769–784. https://doi.org/10.2307/1313099.
Polis, G.A., W.B. Anderson, and R.D. Holt. 1997. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28 (1): 289–316. https://doi.org/10.1146/annurev.ecolsys.28.1.289.
Polis, G.A., A.L.W. Sears, G.R. Huxel, D.R. Strong, and J. Maron. 2000. When is a trophic cascade a trophic cascade? Trends in Ecology & Evolution 15 (11): 473–475. https://doi.org/10.1016/S0169-5347(00)01971-6.
Potapov, A.M., U. Brose, S. Scheu, and A.V. Tiunov. 2019. Trophic position of consumers and size structure of food webs across aquatic and terrestrial ecosystems. The American Naturalist 194 (6): 823–839. https://doi.org/10.1086/705811.
Post, D.M. 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83 (3): 703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO2.
Post, D.M., C.A. Layman, D.A. Arrington, G. Takimoto, J. Quattrochi, and C.G. Montaña. 2007. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152 (1): 179–189. https://doi.org/10.1007/s00442-006-0630-x.
Power, M., G.M. Klein, K.R.R.A. Guiguer, and M.K.H. Kwan. 2002. Mercury accumulation in the fish community of a sub-arctic lake in relation to trophic position and carbon sources. The Journal of Applied Ecology 39 (5): 819–830. https://doi.org/10.1046/j.1365-2664.2002.00758.x.
Power, M.E., A. Sun, G. Parker, W.E. Dietrich, and J.T. Wootton. 1995. Hydraulic food-chain models. BioScience 45 (3): 159–167.
Quay, P., R. Sonnerup, T. Westby, J. Stutsman, and A. McNichol. 2003. Changes in the 13C/12C of dissolved inorganic carbon in the ocean as a tracer of anthropogenic CO2 uptake. Global Biogeochemical Cycles 17(1): 4–1–4–20. https://doi.org/10.1029/2001GB001817.
R Core Team. 2021. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. https://www.R-project.org/.
Rennie, M.D., W.G. Sprules, T.B. Johnson, and C. Kraft. 2009. Factors affecting the growth and condition of lake whitefish (Coregonus clupeaformis). Canadian Journal of Fisheries and Aquatic Sciences 66 (12): 2096–2108. https://doi.org/10.1139/F09-139CODEN:CJFSDX.
Ricker, W.E. 1930. Feeding habits of speckled trout in Ontario waters. Transactions of the American Fisheries Society 60 (1): 64–72. https://doi.org/10.1577/1548-8659(1930)60[64:FHOSTI]2.0.CO.
Roach, K.A., J.H. Thorp, and M.D. DeLong. 2009. Influence of lateral gradients of hydrologic connectivity on trophic positions of fishes in the Upper Mississippi River. Freshwater Biology 54: 607–620. https://doi.org/10.1111/j.1365-2427.2008.02137.x.
Rohtla, M., M. Vetemaa, K. Urtson, and A. Soesoo. 2012. Early life migration patterns of Baltic Sea pike Esox lucius. Journal of Fish Biology 80 (4): 886–893. https://doi.org/10.1111/j.1095-8649.2012.03226.x.
Romanuk, T.N., A. Hayward, and J.A. Hutchings. 2011. Trophic level scales positively with body sizes in fishes. Global Ecology and Biogeography 20 (2): 231–240. https://doi.org/10.1111/j.1466-8238.2010.00579.x.
Sánchez-Hernández, J., and P.-A. Amundsen. 2018. Ecosystem type shapes trophic position and omnivory in fishes. Fish and Fisheries 19 (6): 1003–1015. https://doi.org/10.1111/faf.12308.
Sánchez-Hernández, J., A.P. Eloranta, A.G. Finstad, and P. Amundsen. 2017. Community structure affects trophic onotogeny in a predatory fish. Ecology and Evolution 7 (1): 358–367. https://doi.org/10.1002/ece3.2600.
Saint-Jacques, N., H.H. Harvey, and D.A., Jackson. 2000. Selective foraging in the white sucker (Catostomus commersoni). Canadian Journal of Zoology 78 (8): 1320–1331. https://doi.org/10.1139/cjz-78-8-1320.
Sanders, D., E. Thébault, R. Kehoe, and F.J. Frank van Veen. 2018. Trophic redundancy reduces vulnerability to extinction cascades. Proceedings of the National Academy of Sciences 115 (10): 2419–2424. https://doi.org/10.1073/pnas.1716825115.
Scharf, F.S., F. Juanes, and R.A. Rountree. 2000. Predator size-prey size relationships of marine fish predators: Interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Marine Ecology 208 (229–2): 48. https://doi.org/10.3354/meps208229.
Schindler, D.E., and M.D. Scheuerell. 2002. Habitat coupling in lake ecosystems. Oikos 98: 177–189.
St George, J.R., S.D. Petersen, J.D. Roth, S.H. Ferguson, and D.J. Yurkowski. 2023. Habitat coupling dynamics of mobile consumers along a freshwater and marine resource gradient in a sub-Arctic estuarine system. Estuarine, Coastal and Shelf Sciences 292: 108449. https://doi.org/10.1016/j.ecss.2023.108449.
Stewart, D.B., N.J. Mochnacz, J.D. Reist, T.J. Carmichael, and C.D. Sawatzky. 2007. Fish diets and food webs in the Northwest Territories: Arctic grayling (Thymallus arcticus). Fisheries and Oceans Canada, Winnipeg.
Svanbäck, R., M. Quevedo, J. Olsson, and P. Eklöv. 2015. Individuals in food webs: The relationships between trophic position, omnivory and among-individual diet variation. Oecologia 178 (1): 103–114. https://doi.org/10.1007/s00442-014-3203-4.
Thiemann, G.W., S.J. Iverson, and I. Stirling. 2008. Variation in blubber fatty acid composition among marine mammals in the Canadian Arctic. Marine Mammal Science 24 (1): 91–111. https://doi.org/10.1111/j.1748-7692.2007.00165.x.
Tiemann, J.S.D.P., M.L. Wildhaber. Gillette, and D.R. Edds. 2004. Effects of lowhead dams on riffle0dwelling fishes and macroinvertebrates in a midwestern river. Transactions of the American Fisheries Society 133 (3): 705–717. https://doi.org/10.1577/T03-058.1.
Vander Zanden, M.J., M.K. Clayton, E.K. Moody, C.T. Solomon, and B.C. Weidel. 2015. Stable isotope turnover and half-life in animal tissues: A literature synthesis. PLoSOne 10 (1): e0116182. https://doi.org/10.1371/journal.pone.0116182.
Vesin, J-P, W.C. Leggett, and K.W. Able. 1981. Feeding ecology of capelin (Mallotus villosus) in the estuary and western gulf of St. Lawrence and its multispecies implications. Canadian Journal of Fisheries and Aquatic Sciences 38(3): 257–267. https://doi.org/10.1139/f81-037.
Viljanen, M. 1983. Food and food selection of cisco (Coregonus albula L.) in a dysoligotrophic lake. Hydrobiologia 101(1–2): 129–138. https://doi.org/10.1007/BF00008665.
Vincent, C., M. Huon, F. Caurant, W. Dabin, A. Deniau, S. Dixneuf, L. Dupuis, J.-F. Elder, M.-H. Fremau, S. Hassani, A. Hemon, J. Karpouzopoulos, C. Lefeuvre, B.J. McConnell, S.E.W. Moss, P. Provost, J. Sptz, Y. Turpin, and V. Ridouz. 2017. Grey and harbour seals in France: Distribution at sea, connectivity and trends in abundance at haulout sites. Deep-Sea Research Part II: Tropical Studies in Oceanography 141: 294–305. https://doi.org/10.1016/j.dsr2.2017.04.004.
Wilder, D.G. 1951. A comparative study of anadromous and freshwater populations of brook trout (Salvelinus fontinalis (Mitchill)). Journal of the Fisheries Research Board of Canada 9: 169–203.
Winemiller, K.O. 1990. Spatial temporal variation in tropical fish trophic networks. Ecological Monographs 60 (3): 331–367. https://doi.org/10.2307/1943061.
Włodarska-Kowalczuk, M., M. Aune, L.N. Michel, A. Zaborska, and J. Legeżyńska. 2019. Is the trophic diversity of marine benthic consumers decoupled from taxonomic and functional trait diversity? Isotopic niches of Arctic communities. Limnology and Oceanography 64 (5): 2140–2151. https://doi.org/10.1002/lno.11174.
Woodland, R.J., F.Y. Warry, V. Evrard, R.H. Clarke, P. Reich, and P.L.M. Cook. 2016. Niche-dependent trophic position distribution among primary, secondary and tertiary consumers. Oikos 125 (4): 556–565. https://doi.org/10.1111/oik.02486.
Yurkowski, D.J., S.H. Ferguson, C.A.D. Semeniuk, T.M. Brown, D.C.G. Muir, and A.T. Fish. 2016. Spatial and temporal variation of an ice-adapted predator’s feeding ecology in a changing Arctic marine ecosystem. Oecologia 180 (3): 631–644. https://doi.org/10.1007/s00442-015-3384-5.
Yurkowski, D.J., N.E. Hussey, A.T. Fish, K.L. Imrie, R.F. Tallman, and S.H. Ferguson. 2017. Temporal shifts in intraguild predation pressure between beluga whales and Greenland halibut in a changing Arctic. Biology Letters 13 (11): 20170433. https://doi.org/10.1098/rsbl.2017.0433.
Yurkowski, D.J., N.E. Hussey, S.H. Ferguson, and A.T. Fisk. 2018. A temporal shift in trophic diversity among a predator assemblage in a warming Arctic. Royal Society Open Science 5 (10): 180259.
Acknowledgements
We thank Manitoba Hydro, North/South Consultants Inc., the Churchill Northern Studies Centre, and local fishers in Churchill for help in the collection of samples. Thanks also to staff from Fisheries and Oceans and the Assiniboine Park Zoo lab for their help with lab work. This work was supported by the The Assiniboine Park Zoo, Fisheries and Oceans Canada, University of Manitoba, Manitoba Hydro, The W. Garfield Weston Foundation, and the Northern Scientific Training Program.
Funding
Manitoba Hydro,University of Manitoba,W. Garfield Weston Foundation,Northern Scientific Training Program
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Communicated by Jill A. Olin
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
St. George, J.R., Petersen, S.D., Roth, J.D. et al. Trophic Structure and a Temporal Shift in Trophic Diversity of Mobile Consumers in a Subarctic Estuary. Estuaries and Coasts 47, 551–566 (2024). https://doi.org/10.1007/s12237-023-01291-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01291-x