Abstract
Changes in freshwater flow to estuaries can cause a suite of ecosystem impacts including eutrophication and alterations to plant communities, zooplankton populations, and other biota. In southwest Florida, historical manipulation of freshwater flow due to development, canals, and drainage ditches is pervasive. Because there are estuaries with reduced, increased, and relatively natural freshwater flow, this region presents an ideal system to study how these changes relate to downstream fish abundances. We used a 20-year trawl dataset focused on juvenile and small-bodied fish from three mangrove-lined sub-estuaries with contrasting flow conditions in southwest Florida’s Ten Thousand Islands to identify important environmental and temporal variables influencing fish populations. We used generalized additive models to investigate total fish abundance, species richness, diversity, and the abundances of 23 ecologically, recreationally, or commercially important species and describe their relationships with important environmental and temporal variables. While salinity and temperature had species-specific relationships with fish abundances, seasonality, interannual variability, and sub-estuary were more closely related to fish than salinity and temperature in most cases. Only 8 of 23 species responded most strongly to temperature, and only 1 species responded most strongly to salinity. This suggests that for most species in our study, temporal factors such as timing of spawning and recruitment variability had stronger relationships with the structure of fish populations than changes in freshwater flow. This work quantified how changes in freshwater flow, using salinity as a proxy, may relate to downstream fish abundances and therefore the potential implications of planned watershed restoration that is part of the Comprehensive Everglades Restoration Plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater flow to estuaries throughout many parts of the world has been directly changed through watershed development, agricultural use, and water management (Drinkwater and Frank 1994; Gillanders and Kingsford 2002; Erwin 2009; Chilton et al. 2021). Some anthropogenic alterations result in more freshwater being delivered, whereas other changes reduce flow or modify the timing of freshwater delivery. Alterations of freshwater flow directly affect aspects of the estuarine environment such as salinity, nutrients, and turbidity, which may in turn impact other parts of the ecosystem such as primary producers, zooplankton abundances, and the critical juvenile life stages of many fish that utilize estuaries as nurseries (Estevez 2002; Gillanders and Kingsford 2002; Lorenz 2014; Chilton et al. 2021). Understanding the impacts of freshwater flow on estuarine ecosystems will become a more urgent information need as coastal human populations continue to rapidly grow, demands on freshwater resources become more competitive, and attempts are made to restore damaged estuaries. Furthermore, climate change impacts including sea level rise as well as altered rainfall and storm events will result in more or less flow to many areas than has been experienced historically (Gillanders and Kingsford 2002; Erwin 2009; Krauss et al. 2011; Chilton et al. 2021).
Generalizations from the large number of studies on the effects of altered freshwater flow to estuaries have been hampered by several challenges. For example, estuarine flow includes landscape-scale causes and effects making experimental manipulations seldom possible, individual environmental variables difficult to study in isolation, and comparable control ecosystems lacking. Further complicating matters, freshwater flow is often correlated with multiple variables such as seasonal rainfall, temperature, nutrient loads, and dissolved oxygen (Kanandjembo et al. 2001; Idelberger and Greenwood 2005; Palmer et al. 2011; Kendall et al. 2022). These seasonal correlations make discerning which variables are relatively more influential on fishes difficult to disentangle. Some studies have confounding variables like those in river systems comparing salinity effects at different distances from the ocean (Ley et al. 1999; Stevens et al. 2010; Palmer et al. 2015). In addition, most studies are short term, and are therefore limited by the natural interannual variability in rainfall or recruitment that takes place, lacking a sufficient multi-year time series to appreciate the full range of local conditions important in most systems (Estevez 2002; Palmer et al. 2015). Furthermore, every system is different (Estevez 2002; Jenkins et al. 2010; Chilton et al. 2021). The direction (more vs. less freshwater), magnitude, and timing of changes in flow relative to natural conditions differ uniquely among estuaries and water management practices in their watersheds. Hydrologic impacts documented in one ecosystem will not necessarily be the same in another.
Another issue that makes general conclusions about the impact of alterations to freshwater flow challenging is the differences in species composition among estuaries as well as the particular aspects of the community that are the focus of each study. The choice of study organisms will dictate the results because species differ in their sensitivity to salinity (Irlandi et al. 1997; Patillo et al. 1997; Serafy et al. 1997; Alber 2002). Some researchers focus on effects on the whole fish community such as fish abundance or diversity, whereas other studies focus on particular species, life-stage, or species groups such as anadromous fishes (Kanandjembo et al. 2001; Estevez 2002; Shirley et al. 2004). The environmental variables that researchers seek to relate to fish communities also differ among studies. Some focus on salinity or temperature at the time of the biological sampling; others use longer term averages or measures of variability in the time leading up to the sample (Estevez 2002; Faunce et al. 2004; Kendall et al. 2022). Collectively, these issues mean that broader taxonomic, geographic, and temporal inference from existing studies of altered flow to estuaries is limited. Each specific situation and condition must be investigated using data and analytical approaches that reduce these limitations.
The Ten Thousand Islands area of southwest Florida, USA, has several attributes that alleviate many of the above concerns. Watershed development and canal construction in the 1960s severely altered freshwater flow in the area, which has resulted in three physically similar estuaries with contrasting flow conditions making it an ideal system to understand the impacts of anthropogenic manipulations of hydrology. These man-made alterations have resulted in one estuary receiving 10–100 times the annual freshwater flow of its neighbors, while simultaneously diverting flow from a neighboring estuary that now only receives 1–2% of all freshwater entering these estuaries. The remaining estuary serves as a reference with the least impact to freshwater flow (Shirley et al. 2004; Booth et al. 2014). These three mangrove-lined bays serve as a nursery area for many recreationally or commercially important fishery species, and have been monitored since 2000 through water quality and trawl surveys by the Rookery Bay National Estuarine Research Reserve (RBNERR). This 20-year time series encompasses varying environmental conditions including hurricanes, abnormally wet and dry years, and abnormally warm and cold years, which enables examination of fish abundances with respect to freshwater flow across a multitude of conditions. This system and its fish and water quality monitoring data provide an excellent opportunity to understand how changes in freshwater flow affect juvenile and small-bodied fish abundances (Shirley et al. 2004; Kendall et al. 2022; NOAA NERRS 2020).
Adding to the importance and urgency of understanding the influence of the various flow regimes in these sub-estuaries is the 2022 culmination of a massive watershed restoration endeavor that is part of the Comprehensive Everglades Restoration Program (U.S. Army Corps of Engineers 2004; South Florida Ecosystem Restoration Task Force 2020; Fig. 1a). The objective is to restore and enhance wetlands by reducing over-drainage and moderate the large salinity fluctuations caused by point discharge through a canal system (Wingard and Lorenz 2014). Restoration efforts, which will ultimately include plugging nearly 40 mi of canals and removal of nearly 300 mi of roads, have been underway since 2007, but the most critical, such as the plugging of the Faka Union canal, had not occurred at the time the trawl surveys were collected (Booth and Knight 2021). Understanding the current state of fish abundance in response to freshwater flow can help set downstream expectations following watershed restoration and potentially provide recommendations on managed flow rates.
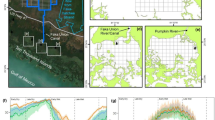
Study area (a). Daily mean salinity + / − range (averaged across all study years) (b) and temperature (c) from the water quality monitoring stations noted in (a). Dashed lines in (b) and (c) represent the breaks between seasons. Note that (b) and (c) have been modified from Kendall et al. (2022)
In this study, we aimed to understand the relationships between juvenile and small-bodied fish abundance and freshwater flow, using salinity as a proxy, in three bays of southwest Florida that have been differentially impacted by human manipulation of watersheds. Fish community structure in this study system and assemblage-level relationships with salinity were evaluated previously by Kendall et al. (2022). Here, we focus on abundances of individual species and relationships with salinity and other variables. We used a long-term dataset focused on juvenile fish from three different watersheds and their sub-estuaries in RBNERR. Specifically, our goals were to (1) identify important environmental (salinity, temperature) and temporal (seasonal, annual) variables influencing total fish abundance, species richness, diversity, and abundances of a suite of ecologically important species; (2) describe the relationships between the important environmental and temporal variables and fish abundances; and (3) understand the potential impact of anticipated changes in flow to the watersheds on fish abundance.
Methods
Study Area
This study was conducted in three semi-enclosed estuarine bays in southwest Florida’s Ten Thousand Islands, RBNERR: Fakahatchee Bay, Faka Union Bay, and Pumpkin Bay (Fig. 1a). Seasons in this region are defined by rainfall with wet and dry periods (Figs. 1b, c and S1–S3). The early dry season lasts from December to February, when temperatures reach their lowest point (Fig. 1c) and salinity is increasing (Fig. 1b). The late dry season is from March to May, when salinity peaks in the bays, reaching values at or above 35 ppt in all three bays (Fig. 1b). Rainfall dramatically increases near the end of May with often daily thunderstorms, which begins the early wet season (June–August). Temperature typically peaks during August of this season (Fig. 1c). Rainfall decreases in early fall, marking the late wet season which runs from September to November. Salinity in the bays reaches its lowest point early in this season in all three bays although the minimum salinity values among bays are quite different (Fig. 1b).
The watersheds leading into each bay have been modified in different ways which has led to major disparities in freshwater flow during the wet seasons. Fakahatchee Bay is considered a more natural reference site since its watershed has been the least altered by the canal construction (Booth and Knight 2021). Freshwater moves to this bay over relatively undeveloped lands as sheet-flow before accumulating into two tidal creeks that discharge into the bay. Salinity in Fakahatchee Bay typically ranges from 12 to 37 ppt (Figs. 1b and S1).
In contrast, Faka Union Bay is the discharge point for the Faka Union Canal, part of a failed suburban development from the 1960s. The roads and canals of this development were designed to quickly drain a watershed much larger than that of Fakahatchee, into a bay that is half the size. As a result, annual freshwater flow to Faka Union Bay is ~ 10 to 100 times higher than neighboring bays (Shirley et al. 2004; Booth et al. 2014) such that ~ 90% of the cumulative freshwater flow reaching the three study bays comes from the Faka Union Canal alone (Booth and Knight 2021). This results in lower and more variable salinity levels in Faka Union Bay than Fakahatchee. During the wet season, minimum salinity is on average 10 ppt lower than in Fakahatchee and ranges from 1 to 20 ppt (Figs. 1b and S2).
At the other extreme is Pumpkin Bay which receives the least freshwater input of the three bays. For Pumpkin Bay, most of the freshwater sheetflow from the watershed is diverted away from the Pumpkin River toward Faka Union Canal. As a result, Pumpkin Bay has higher and less variable salinity levels than Fakahatchee by 3–5 ppt throughout the year, except during the peak of the dry season when all three bays experience full-strength seawater or even hypersaline conditions (Booth et al. 2014; Figs. 1b and S3). In contrast to salinity, water temperatures are much more consistent among the three bays and typically range from 17 to 34 °C (Fig. 1c).
All bays are fringed with mangroves and share a similar tidal range (~ 1 m), distance to the ocean (~ 6 km), and substrate (sand, mud, shell hash, oyster bar mosaic). However, algae and sponge abundance, which can provide structure for fish, differ among the bays. Algae volume is 4 times more abundant in Fakahatchee and Pumpkin Bays than Faka Union and sponge volume is 14 times more abundant in Fakahatchee and 4 times more abundant in Pumpkin than in Faka Union Bay (Kendall et al. 2022). Salinity within the bays is well mixed and does not vary widely spatially (Soderqvist and Patino 2010).
Trawl Data
A long-term trawl-based monitoring program focused primarily on juvenile fish began at RBNERR in 2000. Four replicate trawls were collected in each bay (Fakahatchee, Faka Union, Pumpkin) every month, with a gap from July 2013 to December 2015. Sites were selected randomly from a grid of 175 × 175 m cells overlaid on a map of each bay (Fig. 1a). Trawls were collected during daylight within 2 h of high tide using a 6-m-wide otter trawl with 38-mm mesh and a 3.2-mm bag liner that was pulled into the current for a target distance of 0.19 km. Actual tow distance was measured for each trawl. All captured fish were identified to species, except mojarras (Eucinostomus spp.), anchovies (Anchoa spp.), and whitings (Menticirrhus spp.). Fish were then counted, measured (up to 20 individuals per species), and released.
The four monthly trawls were combined into a single sample representing each bay for a given month to avoid pseudo-replication. Response variables including total fish abundance, species richness, species diversity, and individual species abundance were calculated from these monthly samples. Total fish abundance was calculated as the total number of fish from all species across the four trawls in each month. Species richness was calculated by totaling the number of unique species present in each trawl and averaging across the four trawls. The Shannon Diversity Index was calculated for each trawl and averaged across the four trawls. In addition to these summary metrics, the abundances of 23 individual target species or genera were also studied (Table 1). These species were selected because they contributed to large differences in the fish community among bays and/or seasons in the companion study by Kendall et al. (2022) or earlier work (Carter et al. 1973; Colby et al. 1985; Browder et al. 1986; Shirley et al. 2004), are recreationally/commercially important, or are threatened species. In addition, the selected species had to be present in > 10% of the trawls to enable robust statistical analyses.
Explanatory Variables
To characterize the environment during each monthly sample, we explored a suite of possible explanatory variables using in situ water quality data collected at each trawl location and nearly continuous water quality monitoring data collected at fixed stations in each bay (Fig. 1a; NOAA NERRS 2020). Temperature and salinity were measured at each trawl site with a YSI (Yellow Springs Instruments). Importantly, salinity served as a proxy for alterations to freshwater flow. While salinity (or temperature) itself can influence fish, the variability or extremes of salinity (or temperature) can also have an effect (Lorenz 1999; Faunce et al. 2004; Kendall et al. 2022). In addition, while the salinity or temperature at the time of the trawl may affect fish abundance, the environmental conditions prior to the trawl may also be important (Estevez 2002). To capture these environmental conditions, we calculated the mean, minimum, maximum, standard deviation, and range of salinity and temperature during various time intervals prior to each trawl sample using the continuous water quality data (Fig. 1a). Time intervals considered include 1, 3, 7, 14, 28, 56, and 84 days before the trawl sample. Purely temporal variables were also investigated. Interannual and seasonal variability were assessed by including the year and month the sample was collected as possible explanatory variables. To account for any differences inherent to each bay that were not measured or included in one of the other environmental variables, bay was also included as an explanatory variable. Trawls with missing explanatory variables were excluded.
Identifying Important Explanatory Variables
We used generalized additive models (GAMs) to identify important explanatory variables for each fish response variable because they can fit non-linear relationships such as cyclical seasonal patterns and interannual variability. The identification of important variables indicated if salinity, a proxy for freshwater flow, influenced juvenile fish abundance, while accounting for other aspects of the environment that also likely had an influence. All statistical analyses were conducted in R version 4.1.0 (R Core Team 2021). GAMs were fit with all possible combinations of explanatory variables, excluding those combinations with highly correlated variables (defined here as Pearson’s r > 0.7), using the mgcv package (Dormann et al. 2013; Wood 2017). The inclusion of correlated variables in a single model complicates inference about which is important given their similar relationship with the response variable (Dormann et al. 2013). Therefore, only a single salinity variable and/or single temperature variable was allowed in each model. In addition, variance inflation factors were calculated for each permissible combination of explanatory variables to check for multicollinearity, and all were < 3.
In the GAMs, non-linear relationships were estimated for the year and month variables. Specifically, we used thin plate regression splines to describe the response variable’s relationship with year, and cyclic cubic regression splines to describe the relationship with month because they best represent seasonal cycles. For month, we set knots at 0 and 12, so that January and December would not be forced to have the same exact effect. Residual temporal auto-correlation was insignificant when year and month variables were present. Only linear relationships were estimated for the environmental variables to prevent overfitting, especially when modeling individual species that were present in a low number of trawls. Models for species richness and diversity were fit with a normal distribution whereas all models for fish abundance (total and individual species) were fit using a negative binomial distribution, linking the response variables to the explanatory variables on a log scale.
Although tow length of trawls was generally consistent, an offset for effort was included in all models of fish abundance to account for any effects of variable tow distance. Effort was calculated as the total trawl distance for each sample’s set of trawls. Effort was then standardized by dividing by the average effort value across all samples and log-transforming. For richness and diversity, we did not attempt to adjust for effort in the same way because those metrics can vary non-linearly with sample size and increased effort does not necessarily yield more species or greater diversity (Gotelli and Colwell 2001). Instead, only trawls with a tow distance within 20% of the average tow distance for a single trawl (0.15–0.22 km) were used in models for species richness and diversity to remove those that could bias the analysis.
We fit all models with all permissible combinations of explanatory variables to each response variable and then used Akaike’s information criterion (AIC) to identify the best performing models. AIC scores the relative fit among models, while penalizing for overfitting. The model with the lowest score is considered the best; however, models with an AIC score within 2 of the lowest AIC score are considered similar in performance and fit (Burnham and Anderson 2002). Therefore, all models with an AIC within 2 of the lowest AIC score were deemed the set of models that best explained each response variable (i.e., each individual response variable can have several ‘best’ models). Any explanatory variables present in the best models were defined as important for subsequent parts of the analysis. Percent deviance explained, a measure of how well the model explains the variation in the trawl data, was averaged across all the best models for each response variable.
To understand the importance of each individual explanatory variable (e.g., mean salinity during the 1 day prior to the trawl), we calculated the proportion of best models for each fish response variable that each explanatory variable was present in. We also sought to understand the overall importance of the various aspects of salinity and temperature (e.g., mean) in explaining fish abundance, so we calculated the proportion of the best models that each environmental variable occurred in for each species, regardless of time interval. These proportions were then averaged across all individual species to create a composite value of each environmental variable’s importance collectively for this group of species. Similarly, to understand the time frame over which salinity and temperature best explained abundance of each species, we calculated the proportion of the best models in which environmental variables measured over the same time interval occurred (e.g., 1 day), regardless of which aspect of salinity or temperature it was. These proportions were then averaged across all species to create a composite value of each time interval’s importance collectively for this group of species.
To combine the results of all the top models for each fish response variable, we averaged the models using the model.avg() function from the MuMIn package, weighting each by their AIC score to give better performing models more weight (Burnham and Anderson 2002; Barton 2020). The model averaged coefficients used to calculate effect size (more details in “Relationships between Important Explanatory Variables and Fish” section) were included from only the models where the explanatory variable was present, also known as the subset average. Because we had many similar explanatory variables (mean salinity over 1 day, mean salinity over 7 days), often a set of top models would include each similar variable only once. Using the full model averaged coefficients would assign a value of zero when including a model where the explanatory variable was not present, biasing the averaged coefficients toward zero. Therefore, the subset average was preferred.
Relationships between Important Explanatory Variables and Fish
After important explanatory variables were identified, we next examined the direction (positive or negative) and magnitude (or effect size) of the relationships between those variables and the response variables. This enabled us to understand how freshwater flow and other aspects of the environment affected fish abundance. To assess the effect size of salinity and temperature, we first used the subset model-averaged coefficients to calculate proportional change in fish abundance (or species richness and diversity) between the minimum and maximum observed values for each of the important environmental variables in Fakahatchee Bay, our relatively natural reference system (Table S1). This proportional change indicates how big a change there is in the response variable across the range of values of the explanatory variable (e.g., mean salinity) in a natural system, Fakahatchee Bay. For example, if the model output indicated that abundance of Mojarras increased 3.7 times over the range of salinity observed in Fakahatchee (12.8 to 38.9 ppt), 3.7 represented the effect size of salinity. The modeled effects of the environment, per unit (i.e., 1 °C or ppt), are the same in Faka Union and Pumpkin Bays, but the total potential effect may change due to differing ranges of explanatory variables in those bays.
To assess the effects of bay, month, and year in isolation, we predicted the response across all values of each of these three variables individually from each of the best models and averaged the predictions. To make predictions for these individual terms, models require a constant value for all other variables. In this case, all other continuous explanatory variables were held at their average value (salinity, temperature) and all other categorical variables at a constant reference value to reflect average conditions, during the middle of the monitoring program from the natural reference bay (i.e., bay as Fakahatchee, year as 2009, and month as June). For example, to estimate the effect of month in isolation, we predicted the number of fish in each month from January to December in Fakahatchee Bay in 2009. Finally, because the number of fish varies widely across species (e.g., 100’s of mojarras, 10’s of gray snapper), values were scaled to the maximum predicted value for each species to facilitate visual comparison. For example, a value of 0.25 indicates abundance is 75% lower than the maximum abundance.
While the plots for this section accomplish our objective of understanding the influence of bay, month, and year in isolation, it is important to recognize that actual values of fish abundance are the product of all variables, including those not tested here, acting in concert. Therefore, to put the effects of individual variables into context, we also plotted the observed mean values of fish abundance from the trawls for comparison.
Comparing Effect Size Across Explanatory Variables
In addition, we sought to compare the relative strength of the effects of bay, month, and year to those of the environmental variables, to gauge the impact of restoration of natural freshwater flow to this system, compared to other factors not influenced by restoration. For this, we calculated the proportional change in the response variable between the highest and lowest predicted values for each level of bay, month, and year. For example, mojarras were 8.7 times more abundant in September than in April. These effect sizes were then compared to those calculated for salinity and temperature.
Results
Over the 20 years of the trawl survey, 2664 trawls were collected and 1659 of those (62%) had a complete set of explanatory variable data for analysis. These individual trawls were combined into 419 monthly samples composed of 146 from Fakahatchee, 165 from Faka Union, and 108 from Pumpkin Bay. Of these monthly samples, all but 12 included all four trawls. In Fakahatchee, Faka Union, and Pumpkin Bay, respectively, 76, 58, and 114 months of the possible 222 months spanning the monitoring program were not included in the analysis due to missing explanatory variables (Figs. S1–S3). More are missing from Pumpkin Bay due to the lack of a continuous water quality data logger prior to 2004. The 23 species we selected to examine individually (Table 1) represented 98% of the total fish collected in these samples.
Important Explanatory Variables
We tested 10,952 possible combinations of explanatory variables in our analysis of fish data from trawl catch. The variables bay, month, and year were important for nearly all fish response variables (Fig. 2a). For total fish abundance, 3 models performed equally well and explained an average of 46% of the deviance. We found that maximum salinity during 1, 3, or 7 days before the trawl samples and mean temperature during the 28 days before the samples were important environmental variables for total fish abundance. For species richness, 7 models performed equally well and explained 43% of the deviance. The variability of salinity measured over longer time intervals (56–84 days) prior to the trawl samples were more important for species richness, as was mean temperature during 28 and 56 days before the sample. For fish diversity, 33 models performed equally well, but explained an average of only 33% of the deviance. Standard deviation measured during the 84 days before the trawl was the only important salinity variable, but almost any temperature variable was suitable.
a Frequency of explanatory variables in the best models for each response variable. b Frequency of aspects of salinity and temperature in the best models averaged across all individual species, regardless of time interval. c Frequency of time intervals of salinity and temperature variables in the best models averaged across all species, regardless of which specific aspects of salinity and temperature occurred. Error bars in (b) and (c) represent standard error
For individual species, not only did the number of equally good models vary widely, but so did deviance explained, and the types of environmental predictors that were important (Table 1; Fig. 2a). Four species had especially low PDE (only 18–23%), anchovy species, fringed flounder, gray snapper, and inshore lizardfish, which indicates that other variables not considered here are important drivers of their abundance. In contrast, five species had high levels (60–76%) of the deviance in abundance explained by variables considered in this analysis. These were gafftopsail sea catfish, spotted seatrout, mojarra species, pigfish, and gulf flounder. The remaining 12 species had intermediate PDE values between 30 and 59%. The abundance of some species was explained best by only one or two optimal models and a single aspect of salinity. For example, maximum salinity over 14 days before the trawl was the only important salinity variable for lined sole, and mean salinity during the 84 days before the trawls was the only important salinity variable for mojarra species. In contrast, the abundance of some species was not strongly related to any single salinity variable such that any of several salinity variables performed similarly well and many models performed equally. For example, 31 out of 36 salinity variables similarly described the abundance of sheepshead and 36 models were equally good. Similarly, code goby abundance was well described by any aspect of salinity except standard deviation and 38 models were equally acceptable.
Considering the target species collectively and which aspect of salinity (e.g., mean vs. standard deviation) is most important, regardless of the time interval it was measured, we found that each aspect of salinity, except range, was selected as an important explanatory variable at similar rates (Fig. 2a, b). For most species, multiple aspects of salinity were selected as important variables. If we focus on only the time interval of the salinity predictor prior to the sample (e.g., 1 day vs. 3 days) regardless of its aspect, we find that predictors during the 84 days prior to the sample are most commonly selected and are present in 30% of the top models (Fig. 2c).
There was less variation among species with respect to the number of important temperature variables compared to salinity. For most species, abundance related best to only a single temperature variable, regardless of the number of equally suitable models (Fig. 2a). Considering the target species collectively, mean temperature was by far the most commonly related aspect of temperature to abundance of target species, present in on average 40% of the best models (Fig. 2b). Similar to salinity, measurements of temperature over longer time intervals were more commonly important variables, with 56 and 84 day measurements selected most (Fig. 2c).
Relationship between Explanatory Variables and Responses
To understand the relationship between important explanatory variables and response variables, we calculated the change in the response between the minimum and maximum observed values of the explanatory variable in Fakahatchee Bay, our most natural reference system (Fig. 3; Table S1). For example, a value of 2 for mean salinity indicates fish abundance doubled over 8.5 to 43 ppt mean salinity (the normal range of salinity values in Fakahatchee bay), whereas a value of 0.5 indicates a fish abundance halved. Bay, month, and year variables appear in both the positive (Fig. 3a) and negative parts of the plot (Fig. 3b), simply as the inverse of each other, so that their relative strength can be compared to both positive and negative environmental effects.
Effect sizes of important explanatory variables, averaged across the best models where the explanatory variable was present. Each cell represents the proportional change in the response variable between the lowest value and the highest value of the explanatory variable observed in Fakahatchee Bay, with (a) representing positive relationships and (b) representing negative relationships. Warmer colors represent stronger effects while cooler colors represent weaker effects. In (a), three effects were much larger than the rest (> 150 times increase) and are excluded from the color scale to prevent distortion and represented by dark red
Salinity and Temperature Effects
Total fish abundance decreased by a factor of 0.41–0.43 across the range of values of maximum salinity, while diversity decreased by a factor of 0.82 with mean salinity (Fig. 3b; Table S1). Species richness only increased 1.1 times with the range of salinity and standard deviation of salinity. The relationships between individual species’ abundance and salinity variables, proxies for freshwater flow alterations, varied in strength and direction. Positive effects of salinity ranged from 1.1 to 54 times increase in abundance whereas negative effects ranged from reducing abundance by a factor of 0.01–0.95 (Fig. 3a). Some species responded positively to increasing salinity, such as lane snapper, whose abundance increased by 51–54 times with mean salinity. Other species responded negatively, such as bighead searobin, whose abundance changed by a factor of 0.29–0.37 with minimum salinity. Some species had a positive relationship with salinity variability, such as green goby, whose abundance increased 3 times with standard deviation of salinity, while others had a negative relationship, such as gray snapper, whose abundance changed by a factor of 0.36–0.79 with standard deviation of salinity. Some species had only weak relationships with salinity variables, such as sheepshead and code goby.
Generally, temperature had a stronger effect on fish abundance compared to salinity (Fig. 3). Total fish abundance increased 3.7 times across the range of mean temperature values (Table S1). Species richness also increased 1.7–1.8 times with mean temperature, whereas diversity had a very weak relationship with all temperature variables. The direction and strength of the relationship between important temperature variables and individual species’ abundance was highly variable. Positive effects on individual species ranged from 1.7 to 647 times increases in abundance while negative effects ranged from reductions by a factor of 0.02–0.58. Some species responded positively to temperature, such as silver perch whose abundance increased 647 times with mean temperature, spotted seatrout whose abundance increased 34 times with maximum temperature, and lane snapper whose abundance increased 19 times with minimum temperature (Fig. 3a). Other species responded negatively to temperature, such as pigfish, whose abundance changed by a factor of 0.02 with minimum temperature, and gulf flounder, whose abundance changed by a factor of 0.1–0.24 with mean temperature and by a factor of 0.38 with minimum temperature (Fig. 3b). Some species responded positively to temperature variability. For example, sheepshead abundance increased 9–15 times with standard deviation of temperature and range of temperature, whereas others responded negatively to temperature variability, such as pinfish and gafftopsail sea catfish, whose abundance changed by a factor of 0.14 and 0.02, respectively (Fig. 3).
Bay Effects
Bay had an effect on all response variables except inshore lizardfish abundance. Holding all other environmental and temporal variables constant, total fish abundance was lowest in Fakahatchee Bay, higher in Faka Union Bay (1.25 times more fish than Fakahatchee), and highest in Pumpkin Bay (1.8 times more fish than Fakahatchee) (Fig. 4a). Species richness was lowest in Faka Union (0.84 times the species than in Fakahatchee), followed by Fakahatchee then Pumpkin (1.1 times more species than Fakahatchee). Diversity was highest in Fakahatchee Bay, followed by Pumpkin Bay (0.9 times less the diversity of Fakahatchee), with Faka Union Bay having the lowest diversity (0.75 times the diversity of Fakahatchee).
Estimated fish response variables across bays (a) and mean observed values in monthly trawls (b). Predicted responses are calculated for each best model and averaging the results, while holding all environmental variables at their mean, month as June, and year as 2009. Values are scaled to facilitate comparison among species with different absolute abundances
The effect of bay, in isolation of other variables, on abundance varied widely among species (Fig. 4a). Hardhead catfish, gafftopsail sea catfish, silver perch, fringed flounder, code goby, lane snapper, pigfish, Gulf flounder, and bighead searobin were most abundant in Fakahatchee Bay, ranging from 1.09 to 13 times more abundant than in Faka Union Bay and 1.03–5.93 times more abundant than in Pumpkin Bay. Sand seatrout, spotted seatrout, mojarra species, gray snapper, and clown goby were most abundant in Faka Union Bay and ranged from 1.16 to 3.12 times more abundant than in Fakahatchee Bay. Lined sole, anchovy species, sheepshead, pinfish, whiting species, green goby, blackcheek tonguefish, and Gulf pipefish were most abundant in Pumpkin Bay, ranging from 1.09 to 3.2 times more abundant than in Fakahatchee Bay. Sheepshead, pinfish, Gulf pipefish, hardhead catfish, gafftopsail sea catfish, silver perch, fringed flounder, code goby, lane snapper, pigfish, Gulf flounder, and bighead searobin were all least abundant in Faka Union Bay, and ranged from 0.08 to 0.92 times their abundance in Fakahatchee Bay.
When comparing the effect of bay independent of other variables like the environment (Fig. 4a), to the patterns in the observed data (Fig. 4b), we see some differences. For most species, the patterns observed for the effect of bay by itself mirror the patterns in the raw data. However, for bighead searobin, blackcheek tonguefish, gray snapper, green goby, lane snapper, lined sole, and whiting species, these patterns differ (Fig. 4). For example, with all other variables including the environment held equal, lane snappers were most abundant in Fakahatchee Bay. However, they also have a strong positive relationship with mean salinity (Fig. 3), stronger than the effect of bay, which is consistent with lane snappers being most abundant in Pumpkin Bay in the observed data (Fig. 4b). Similarly, green goby was most abundant in Pumpkin Bay when considering all other variables equal, but had a positive relationship with salinity variability, an effect that was stronger than that of bay. Therefore, in the observed data, green goby are most abundant in Faka Union Bay. For the species where the effect of bay in isolation differed from the observed data in the trawls, the species relationship with the environment explained the difference.
Month Effects
All response variables were affected by month in clear patterns except for species richness and abundance of lined sole, sand seatrout, and lizardfish, which were only weakly affected (Fig. 5a). Total fish abundance peaks in August during the early wet season, whereas diversity peaks in April during the dry season. Most species had a strong pattern when considering the influence of season alone, but each was different, such that very few target species peaked in abundance during the same month. Green goby, fringed flounder, hardhead catfish, clown goby, and code goby were most abundant during the early dry season (December–February), whereas whiting species, pinfish, silver perch, Gulf flounder, sheepshead, and pigfish peaked a little later in the late dry season (March–May). The early wet season (June–August) brought peak abundance for Gulf pipefish, gray snapper, gafftopsail sea catfish, and spotted seatrout. Blackcheek tonguefish, mojarra species, lane snapper, bighead searobin, and anchovy species had their highest abundance in the late wet season (September–November).
Estimated fish response variables across months (a) and mean observed values in monthly trawls (b). Predicted responses are calculated for each best model and averaging the results, while holding all environmental variables at their mean, bay as Fakahatchee, and year as 2009. Values are scaled to facilitate comparison among species with different absolute abundances. Species on the y-axis are ordered by month with the highest predicted value
Examining the observed data (Fig. 5b) enables us to understand the seasonal patterns in actual fish abundance. For nine response variables, the patterns were different than the estimated effect of month by itself. For example, whitings are most abundant in March and April, based on the effect of month by itself (Fig. 5a). However, the observed peak is in October. Whitings have a strongly positive relationship with mean temperature, which outweighs the influence of season alone and leads to this difference in patterns. Similarly, sand seatrout did not vary much across months, but had a strong positive relationship with temperature and a strong negative relationship with salinity, which resulted in a peak in abundance in September, even though month by itself did not have a large effect. For all these responses, the difference in patterns between the effect of month in isolation and in the observed data could be explained by the effect of the environment. These differences indicate that factors outside of the environment drive the effect of month.
Interannual Effects
All responses varied widely among years (Fig. 6). The highest total number of fish occurred in 2006, whereas species richness and diversity varied only slightly throughout the study period. The specific years of peak abundance were not aligned among all species. Only two species had a consistent trend in abundance over time throughout the study period: fringed flounder, which increased in abundance by a factor of 48, and gray snapper, which was reduced by a factor of 0.29 throughout the 20-year survey.
Estimated fish response variables across year (a) and mean observed values in monthly trawls (b). Predicted responses are calculated for each best model and averaging the results, while holding all environmental variables at their mean, bay as Fakahatchee, and month as June. Values are scaled to facilitate comparison among species with different absolute abundances. Species on the y-axis are ordered by year with the highest predicted value
Relative Strength of Effects
In comparing the relative strength of the effects of environmental versus temporal explanatory variables, the temporal variables, month and year, had stronger effects on fish abundances overall (Fig. 3). Year, which represents interannual variability and long-term changes in abundance, had the strongest effect on species richness, total fish, lined sole, anchovy species, gafftopsail sea catfish, clown goby, Gulf flounder, blackcheek tonguefish, and inshore lizardfish abundance. Month, which represents seasonality, had the strongest effect on species diversity, mojarra species, pinfish, gray snapper, pigfish, bighead searobin, and Gulf pipefish abundance. Fringed flounder was the only species whose abundance was more strongly affected by bay compared to other important variables. Four species were slightly more strongly affected by temperature variables (green goby, spotted seatrout, code goby, and sheepshead), and only four were much more strongly affected by temperature variables (whitings, hardhead catfish, silver perch, and sand seatrout). Salinity had the strongest effect of any explanatory variable on the abundance of only one species, lane snapper (Fig. 3).
Discussion
Anthropogenic manipulations of freshwater flow can cause a suite of impacts to estuarine fishes (Drinkwater and Frank 1994; Estevez 2002; Chilton et al. 2021). In southwest Florida, development has increased freshwater flow to some mangrove-lined estuaries in the Ten Thousand Islands and decreased it in others, which provides an ideal setting to examine these effects (Shirley et al. 2004; Kendall et al. 2022). We used a 20-year trawl dataset, rare for this region, to understand how manipulations of freshwater flow relate to estuarine fish abundances, species richness, and diversity. While salinity and temperature had a persistent effect on fish abundances, both of these drivers were weak relative to the effect of month and year, which suggests that seasonal cycles and interannual variability are more important than short-term changes in salinity and temperature. Because relationships with salinity serve as a proxy for relationships with changes in freshwater flow, this work suggests that restoration of natural hydrology may have minor impacts on the abundances of many juvenile estuarine fishes in the Ten Thousand Islands region. However, the relationships between freshwater flow and fish abundance may vary estuary to estuary. What occurs in one system may not be the same in another, especially when the range of freshwater flow is highly variable across systems and the results from this study may not extend to other regions.
Salinity and Temperature Effects
Salinity
Both natural and anthropogenic changes to freshwater flow can alter the salinity conditions of coastal estuaries and affect the abundance of estuarine fish (Estevez 2002; Lorenz and Serafy 2006; Tolley et al. 2006; Lorenz 2014; Gandy and Rehage 2017; Rubec et al. 2021). Every fish species or fish variable considered in this study was related to one or more aspects of salinity although the timescale, strength, and direction of this association varied widely. Although many estuarine fishes are adapted to tolerate a euryhaline environment, they often seek to optimize their position in the estuary to maximize fitness directly by seeking physiologically suitable salinity (Sheaves 1996; Serafy et al. 1997; Serrano et al. 2010; Rubec et al. 2021), or through indirect processes such as use of energy sources that are affected by salinity (Montague and Ley 1993; Palmer et al. 2011, 2015; Vinagre et al. 2011; Lorenz 2014; Loh et al. 2017), avoidance of predators or pathogens, or migratory imperatives such as seeking spawning or recruitment habitat (Grange et al. 2000; Kanajembo et al. 2001; Jenkins et al. 2010; Serrano et al. 2010). In this study, aspects of salinity measured over longer time intervals of weeks to months best related to fish abundance. This is broadly consistent with previous studies that examined multiple time periods of environmental conditions prior to collection of fish samples (Lorenz 1999; Faunce et al. 2004).
Across the group of species examined in this study, four measures of salinity (mean, minimum, maximum, and standard deviation) were primarily related to fish abundance and were present in 15–25% of models for each species. This suggests that some aspect of salinity is important for understanding fish abundance, but several measures of salinity can adequately describe the effect. Some species such as clown goby, sand seatrout, and anchovy species responded to maximum and minimum salinity, which could suggest that they are approaching the limits of their salinity tolerance; however, the measured salinity over the 20-year study never surpassed their known tolerances (Patillo et al. 1997). Instead, it is possible that maximum and minimum salinity, which are correlated with other aspects of salinity, are representing the general salinity conditions in the environment, and at least some salinity variable is necessary to adequately explain fish abundance in the models. This could explain why there were many equally good models and equally suitable salinity variables for several species considered here such as sheepshead and code goby. Other fish were related to variability of salinity, such as gray snapper. More variable salinity in nearby Florida Bay has been linked to lower quality food availability for some estuarine fishes (Montague and Ley 1993; Ley et al. 1994). In the present study, a few species such as hardhead catfish and gray snapper responded negatively to increased variability in salinity, whereas others increased in abundance such as silver perch, pigfish, green goby, and whiting species. This confirms that many estuarine species are more or less tolerant of salinity values but that each should be considered on an individual basis when seeking to predict how changes in salinity following watershed restoration may impact their abundance.
Although some measure of salinity was always needed to describe fish abundance well, it is important to note that for all but lane snapper, the effect of salinity, was weaker than for other explanatory variables like temperature, month, and year. This finding is consistent with several other studies that compared the relative effect size of salinity on estuarine fishes compared to other, more influential variables such as season (Idelberger and Greenwood 2005), turbidity (Blaber and Blaber 1980), and depth (Faunce et al. 2004). This is further evidence that even though the species evaluated here responded to different measures of salinity, the effects are small given the salinity values observed in these bays. For some species, such as sheepshead and code goby, the effect of salinity was so small that any salinity variable equally well described their abundance. These results suggest that restoring freshwater flow to pre-development conditions in these bays may not have a major impact on the abundance of fishes considered here. It is important to note that these relationships are complex and often location specific. Other estuaries with more extreme variation in freshwater flow, such as Florida Bay or parts of the Peace River in Charlotte Harbor, observe stronger relationships between freshwater flow and fish abundances than in this study (Faunce et al. 2004; Lee et al. 2016; Stevens et al. 2013). However, in the Ten Thousand Islands, there is still much overlap in the salinity between these three bays and changes to salinity have not reached such extremes.
Temperature
Temperature variables not only had a consistently stronger relationship to fish abundance than salinity overall, but a single aspect of temperature measured at longer time intervals (e.g., mean temperature over 2 to 3 months prior to trawls) was selected in a clear majority of top models and for most species. This long time interval is essentially representative of seasonal cycles of temperature. We suspect that this seasonal aspect of temperature is the driver of abundance rather than temperature per se (Idelberger and Greenwood 2005; “Seasonal effects” section below). In fact, many studies do not include temperature in their analysis of fish communities, but instead include month or season which is a proxy for temperature in most regions (Faunce et al. 2004; Shirley et al. 2004; Tolley et al. 2006; Vinagre et al. 2011; Gandy and Rehage 2017). It is more likely that within-year cycles of abundance are driven by reproduction which occurs in distinct seasons for most taxa (Patillo et al. 1997). Similar results were observed for the community as whole in the multivariate companion study Kendall et al. (2022), where temperature had much stronger correlations with the fish community than salinity.
These findings do not indicate that temperature per se is unimportant for all species; in fact, abundance of gray snapper and pinfish was strongly negatively related to temperature variables measured on timescales as short as 1–3 days. In addition, a few species were better explained by temperature variability, such as fringed flounder, gulf pipefish, sheepshead, pigfish, and pinfish, although still over longer time scales. In Florida Bay, fish density increased with increasing temperature variability (Lorenz 1999). Also of note, seasonal temperature values were consistent throughout the study area and are not suspected to be responsible for differences in fish abundance among the bays.
Bay Effects
Bay was an important variable in nearly all models for every species except for inshore lizardfish. Including bay as an explanatory variable integrates effects on fish abundances from differences among the bays that are not accounted for in variables such as salinity. This can include fixed physical variables such as bay size or perimeter to area ratio which can affect exposure to predators that are associated with mangrove edges such as snook, gray snapper, and goliath grouper (Patillo et al. 1997; Koenig et al. 2007). Bay effects may also encompass differences in habitat ratios among the bays such as percent cover of oyster reefs, macroalgae, and sponges. For example, Faka Union Bay has much less macroalgae and sponge cover than the other two bays (Yokel 1975; Kendall et al. 2022) which affects the structural habitat types available for small fishes. Benthic vegetation is positively correlated with pinfish, code goby, and gulf pipefish abundance (Patillo et al. 1997) and all three species were least abundant in Faka Union Bay, which has the lowest algae volume among bays (Kendall et al. 2022). The effect of bay on total fish abundance could also be explained by its effect on individual species. For example, total fish abundance is highest in Pumpkin Bay, while anchovy species and pinfish, two of the most abundant species, are also most abundant in Pumpkin Bay. It is important to note however, that the bay variable may be capturing some effects of freshwater flow that the salinity measurements we used do not, in addition to those unrelated to freshwater flow, such as area or bottom habitat.
This bay effect also includes potential influences that are subject to different salinities even if the fishes themselves are not, such as benthic prey availability (Ley et al. 1994; Tolley et al. 2006). For example, in Tampa Bay, Florida, higher spotted seatrout abundance was found in areas with more freshwater flow, possibly due to higher food availability or reduced predation risk (Robins et al. 2005; Gillson 2011; Whaley et al. 2016). Similarly, we found spotted seatrout were most abundant in Faka Union, the bay with more freshwater flow, despite increasing in abundance with maximum salinity, which suggests they may be responding to other effects of increased freshwater flow than salinity itself. Similarly, some species that are least abundant in Faka Union Bay compared to Fakahatchee and Pumpkin, such as fringed flounder and bighead searobin, counterintuitively have negative relationships with salinity and therefore are more likely associated with low salinity environments. This result also indicates other drivers in addition to the anthropogenically induced lower salinity conditions are impacting species abundances in Faka Union. The drawback with such an all-encompassing variable is that all these effects are lumped together and the specific influence on fish abundance remains unknown beyond simply differentiating an overall effect among bays.
Although Fakahatchee Bay is the most natural estuary in our study, it does not have the highest values for all fish variables. For example, the total number of fish, per unit effort, is lowest in Fakahatchee compared to Faka Union and Pumpkin Bays. However, Fakahatchee did have the highest diversity, but Faka Union had the lowest. Similarly, variability in the fish community is reduced in areas with freshwater flow in Charlotte Harbor, Florida (Olin et al. 2015). Biodiversity is often used as a measure of ecosystem health and function by more evenly spreading out niche resiliency over a variety of species (Hooper et al. 2005). Ecosystem roles lost by a decline in any one species can be filled by several others. Given this key ecosystem-scale difference among impacted and natural flow conditions of the adjacent estuaries, biodiversity may be a more beneficial target than abundance for monitoring the success and impact of restoration of freshwater flow to Faka Union and Pumpkin Bays.
Seasonal Effects
Total fish abundance, diversity, and the abundance of all species considered here exhibited at least some seasonal variation, some due to seasonal changes in the environment, others due to seasonality itself. For example, considering all other variables equal, there was little difference in sand seatrout abundance across months. However, once we consider the effect of temperature, which has a much stronger effect than month, we see a seasonal pattern in abundance emerge, due to temperature.
Some aspects of the seasonal pattern observed in total abundance and diversity may be explained by seasonal patterns of individual species. For example, total fish abundance is highest during the wet seasons (Shirley et al. 2004), when mojarra species and anchovy species, two very abundant species, peak in abundance. In contrast, diversity peaks in the dry season, when very abundant species are not dominating the community, and is lower during the wet season. Similar patterns are observed in Charlotte Harbor, Florida, where variability in the fish community is reduced in areas with anthropogenically controlled high and variable flow (Olin et al. 2015). This homogenization of the fish community can lead to reduced seasonal effects on fish abundances, which is also observed in the companion study (Kendall et al. 2022), where the fish community in the late and early dry and late and early wet seasons are very similar in Faka Union Bay, which receives more freshwater due to human alterations of the watershed, but the fish community is more distinct across the four seasons in the other bays. This homogenization can be observed in this study via the reduced diversity during the wet season.
Months with peak abundances in the trawl data correspond with the known timing of spawning for 16 of the individual species examined provided that the brief lag until juvenile recruitment is included (Estevez 2002). These include sheepshead, mojarras, pigfish, lane snapper, fringed flounder, gafftopsail sea catfish, gray snapper, pinfish, gulf flounder, code goby, hardhead catfish, inshore lizardfish, lined sole, sand seatrout, silver perch, and whiting species (Yokel 1975; Pattillo et al. 1997, Anderson and Comyns 2013). For example, pinfish spawn in the fall and winter and juveniles recruit to the estuaries in the spring, when we see their abundance in the trawls peak (Yokel 1975; Patillo et al. 1997). Similarly, sheepshead spawn offshore from February to April, with juveniles recruiting shortly after (Patillo et al. 1997), and we found their abundance peak in the trawls in May. In contrast, inshore lizardfish and lined sole abundance varied only slightly throughout the course of the year, which is consistent with their year round spawning behavior (Jones et al. 1978).
Gulf pipefish spawn throughout the year, yet there is a clear peak in abundance in the trawls in July (Ross 2001). Although they spawn year-round, they prefer vegetated habitats. In the bays, algae is most abundant in the late dry and early wet seasons, corresponding with the peak in abundance of gulf pipefish, which indicates their seasonal pattern is related to habitat rather than spawning behavior (Kendall et al. 2022).
The timing of peaks in abundance of the remaining three species did not match known spawning periods, specifically, anchovy species, spotted seatrout, and blackcheek tonguefish (Whitehead 1988; Patillo et al. 1997; Terwilliger and Munroe 1999). For example, blackcheek tonguefish spawn nearly year round or for a prolonged period of time, yet there are clear seasonal peaks in abundance in this study (Reichert and van der Veer 1991). Apparent mismatches with spawning seasons reported in prior research could be due to variability in timing of spawning across regions, habitat shifts at different age classes, some other even more influential factor controlling abundance such as a seasonal predator, or some groups being composed of multiple species (e.g., anchovy species) with differing spawning seasons. Little is known about bighead searobin, green goby, and clown goby spawning, but we found clear seasonal patterns.
Interannual Effects
The long-term monitoring dataset used here was collected consistently over 20 years across a range of environmental conditions (e.g., El Niño–Southern Oscillation, hurricanes). This provides a rare opportunity to perceive and appreciate interannual variability in fish abundance as data collected at such a frequency for such a long period of time is uncommon. Natural variation in recruitment is often a driver of annual fish abundances. Size of spawning populations, food availability, larval transport conditions, drought, extreme cold events, and storms can all influence larval survival and recruitment, and ultimately juvenile abundance in the trawls (Drinkwater and Frank 1994; Greenwood et al. 2006; Stevens et al. 2013; Boucek et al. 2017). In our study, all species abundances varied throughout the study period, although some experienced larger fluctuations than others. Anchovy species and gafftopsail sea catfish had strong changes in abundance across years. Anchovy abundance is strongly related to food supply (zooplankton; Patillo et al. 1997), which also varies naturally through time depending on currents, primary production, and environmental conditions. In this study, the year of highest anchovy abundance (2006) follows a year of elevated chlorophyll concentrations in the bays, which could be linked to higher zooplankton abundance (NOAA NERRS 2020). Anchovy species were one of the most abundant in the study, and their interannual variability may also explain the strong effect of year on total fish abundance. Gafftopsail sea catfish, which were strongly affected by year, exhibited a large catch in the trawls in 2016. Pinfish abundance peaked in 2010 in our study area, as well as adjacent ecosystems (Chacin et al. 2016), which has been suggested to be related to release from predators that died in the extreme cold event of 2009–2010 (Stevens et al. 2016). Gray snapper was the only species to decline in abundance consistently throughout the study period; however, a recent stock assessment suggests the adult population in the Gulf of Mexico was increasing at least from 2010 to 2014 (SEDAR 2018). Other factors must be influencing their continuous decline in catch as juveniles over ~ 20 years in the Ten Thousand Island region. While fringed flounder increased in abundance throughout the study period, little is known about what factors influence their population dynamics. However, for both gray snapper and fringed flounder, the PDE explained by our models was low, 19% and 21%, respectively, and these patterns should be considered with caution. Although identifying the precise causes of interannual variation remain elusive in many cases, it is only possible to perceive the magnitude and variability in these patterns with a long term and consistently collected dataset such as was used here. Shorter term datasets of even a few years would have given the false impression that some species are always dominant when that is not the case.
Relative Strength of Effects
While salinity and temperature were both important to include in models explaining fish abundances, temperature almost always had a stronger effect than salinity, our proxy for changes in freshwater flow. Similarly, previous work in the Everglades’ Florida Bay, which has experienced reduced freshwater flow over the past 60 + years, fish density was more correlated with temperature than salinity (Lorenz 1999). Others have also suggested that temperature was more important for particular species such as sand seatrout and gafftopsail sea catfish abundance than salinity (Ditty et al. 1991; Patillo et al. 1997; Walton et al. 2022).
Although salinity and/or temperature nearly always had an effect on fish abundances, both of these were generally weak relative to the effect of month and year, which suggests that seasonal patterns and interannual variability are more important drivers of fish abundance in this system (Idelberger and Greenwood 2005). Even bay, which may have captured effects of freshwater flow that the salinity variables did not, was generally weaker than month and year. For example, even if it is a dry year with higher salinity, mojarra species are still going to be caught in the wet season after their seasonal recruitment to the estuary. In fact, even the timeframe of influential salinity and temperature variables were generally measured at such a long interval (1–3 months) that they approach representing seasonality. The seasonal basis of spawning cues are tied to the natural cycles in the environment beyond freshwater flow such as day length and were established long before changes to the natural sheet flow of the Big Cypress basin. Similar results were observed in the companion community study Kendall et al. (2022). Differences in the community were more strongly related to temperature than salinity and more strongly related to season than bay. Although season and temperature are linked, and it may be difficult to disentangle the individual effects of each, both had stronger relationships with fish variables than bay and/or salinity. It appears that the recent changes in hydrology will not affect those patterns, especially considering that these are all estuarine species and environmental conditions will remain within the tolerances of most species. In fact, watershed restoration is likely to reduce the range of salinities in Faka Union Bay and make it more similar to Fakahatchee.
The only species where salinity had a stronger effect on abundance than anything else was lane snapper. Although temperature had a stronger effect on sand seatrout abundance than salinity, salinity still had a stronger effect than temporal variables. This suggests that changes in freshwater flow through restoration may have impacts on these two species. Due to its positive relationship with salinity, lane snapper would be expected to increase in abundance in Faka Union Bay as freshwater flow is reduced and decrease in abundance in Pumpkin Bay as freshwater flow is increased with restoration. The opposite could be expected for sand seatrout, which would be expected to decrease in abundance in Faka Union Bay as natural flow is restored due to their negative relationship with salinity.
Future Research
While this work suggests that changes in salinity through restoration of natural hydrology may have minor impacts on juvenile fish abundances, compared to natural seasonal variations in abundance, it is important to note that the species studied here are estuarine species with tolerance for a wide range of salinity conditions. Other species that are less abundant and more sensitive to environmental change would be better indicators of the effect of altering hydrology than those selected in this study. For example, goliath grouper densities have been negatively correlated with salinity in the Ten Thousand Islands, but are not observed enough in the trawls over sand and mud flats for robust analysis (Koenig et al. 2007). However, the species selected in this study do represent 98% of the individuals captured in the survey and therefore the vast majority of the juvenile fish community in these bays. These species make up 25% of the species observed in the trawls; however, the remaining species are represented in part by total fish abundance, species richness, and diversity. Although these rarer species not studied here individually may be more sensitive to changes in salinity and ultimately freshwater flow (Mouillot et al. 2013; da Silva and Fabre 2019), they do not make up a large portion of the fish community.
It is also important to recognize that results here are limited to species that are susceptible to being caught in the trawl gear. Larger and faster species (e.g., juvenile sharks, smalltooth sawfish, juvenile tarpon, juvenile snook) or those that reside on oyster reefs or in the mangrove prop roots (e.g., goliath grouper) are not well sampled with this gear and results should not be generalized for all juvenile fish in the study area. Work in adjacent ecosystems, such as the Shark River Estuary in the Everglades, Caloosahatchee River, and Charlotte Harbor, demonstrated responses of some of these species to human alterations of freshwater flow, including tarpon (Wilson et al. 2019), smalltooth sawfish (Brame et al. 2019), and snook (Rehage et al. 2022), but more work is needed to understand effects on species like these in our three study bays. It is also important to note that this study only focuses on abundances, not biomass, size, or body condition, which can also be impacted by changes in freshwater flow (Crocker et al. 1981).
A few species had poor model performance, such as gray snapper and anchovy species, which suggests that other variables not considered in this study may be of more importance. Dissolved oxygen, bottom type, turbidity, nutrients, and phytoplankton and zooplankton abundance are all variables not considered in this study that could have an impact on these, and all species in this study, and may merit consideration in future work. In addition, interactions between explanatory variables such as bay and month may be an interesting avenue for future research. The impacts of restoration on species not well explained by the variables considered in this work, such as gray snapper, fringed flounder, and inshore lizardfish, must be used with caution.
What Does this Mean for Restoration?
Ultimately this work suggests that salinity changes due to restoration of freshwater flow to these sub-estuaries will have minimal effects on the abundance of most species of juvenile fish, relative to other natural seasonal oscillations in abundance. Two species that can be expected to experience changes in abundances include lane snapper and sand seatrout. Lane snappers are expected to increase in abundance in Faka Union Bay as the canal is plugged and salinity levels in the bay eventually rise. Sand seatrout, however, may decrease in abundance as predicted here based on their negative relationship with salinity. Restoring flow may also make sponge/algae abundance in Faka Union more similar to Fakahatchee Bay and therefore influence the abundance of some species that rely on that structure (Kendall et al. 2022). Changes in abundance of algal habitat may indirectly affect a few species, such as pinfish, gulf pipefish, and code goby, which are commonly associated with that cover type. Despite the expectations suggested here, continued monitoring is critical as flow is altered to confirm that changes are as anticipated, to confirm that environmental flows necessary to sustain ecosystems are maintained, and as climate change further alters temperature and salinity regimes potentially outside of those ranges considered here (Faunce et al. 2004; Jenkins et al. 2010; Vinagre et al. 2011; Boucek et al. 2017; Arthington et al. 2018; Duggan et al. 2019; Van Niekerk et al. 2019).
References
Alber, M. 2002. A conceptual model of estuarine freshwater inflow management. Estuaries 25 (6B): 1246–1261.
Anderson, E.J., and B.H. Comyns. 2013. Distribution, abundance, and feeding habits of juvenile kingfish (Menticirrhus) species found in the North-Central Gulf of Mexico. Gulf of Mexico Science 31 (1): 50–66.
Arthington, A.H., J.G. Kennen, E.D. Stein, and J.A. Webb. 2018. Recent advances in environmental flows science and water management – innovation in the Anthropocene. Freshwater Biology 63: 1022–1034.
Barton, K. 2020. MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn.
Blaber, S.J.M., and T.G. Blaber. 1980. Factors affecting the distribution of juvenile estuarine and inshore fish. Journal of Fish Biology 17: 143–162.
Booth, A.C., L.E. Soderqvist, and M.C. Berry. 2014. Flow monitoring along the western Tamiami Trail between County Road 92 and State Road 29 in support of the Comprehensive Everglades Restoration Plan, 2007–2010: U.S. Geological Survey Data Series 831, 24 p.
Booth, A.C. and T.M. Knight. 2021. Flow characteristics and salinity patterns in tidal rivers within the northern Ten Thousand Islands, southwest Florida, water years 2007–19: U.S. Geological Survey Scientific Investigations Report 2021–5028, 21 p.
Boucek, R.E., M.R. Heithaus, R. Santos, P. Stevens, and J.R. Rehage. 2017. Can animal habitat use patterns influence their vulnerability to extreme climate events? An estuarine sportfish case study. Global Change Biology 23: 4045–4057.
Brame, A.B., T.R. Wiley, J.K. Carlson, S.V. Fordham, R.D. Grubbs, J. Osborne, R.M. Scharer, D.M. Bethea, and G.R. Poulakis. 2019. Biology, ecology, and status of the smalltooth sawfish Pristis pectinata in the USA. Endangered Species Research 39: 9–23.
Browder, J.A., A. Dragovich, J. Tashiro, E. Coleman-Duffie, C. Foltz, and J. Zweifel. 1986. A comparison of biological abundances in three adjacent bay systems downstream from the Golden Gate Estates canal system. NOAA Technical Memorandum NMFS SEFC-185. Miami, FL, USA. 26 pp.
Burnham, K.P., and D.A. Anderson. 2002. Model selection and multimodel inference: A practical information-theoric approach, 2nd ed. New York, NY: Springer.
Carter, M.R., L.A. Burns, T.R. Cavinder, K.R. Dugger, P.L. Fore, D.B. Hicks, H.L. Revells, and T.W. Schmidt. 1973. Ecosystems analysis of the Big Cypress Swamp and Estuaries. United States Environmental Protection Agency. Region 4. Atlanta, Georgia, USA.
Chacin, D.H., T.S. Switzer, C.H. Ainsworth, and C.D. Stallings. 2016. Long-term analysis of spatio-temporal patterns in population dynamics and demography of juvenile pinfish (Lagodon rhomboides). Estuarine, Coastal and Shelf Science 183: 52–61.
Chilton, D., D.P. Hamilton, I. Nagelkerken, P. Cook, M.R. Hipsey, R. Reid, M. Sheaves, N.J. Waltham, and J. Brookes. 2021. Environmental flow requirements on estuaries: Providing resilience to current and future climate and direct anthropogenic changes. Frontiers in Environmental Science 9: 764218.
Colby, D.R., G.W. Thayer, W.F. Hettler, and D.S. Peters. 1985. A comparison of forage fish communities in relation to habitat parameters in Faka Union Bay, Florida and eight collateral bays during the wet season. NOAA Technical Memorandum NMFS SEFC-162. Beaufort, NC, USA. 87 pp.
Crocker, P.A., C.R. Arnold, J.D. Holt, and J.A. DeBoer. 1981. Preliminary evaluation of survival and growth of juvenile red drum (Sciaenops ocellata) in fresh and salt water. Journal of the World Mariculture Society 12 (1): 122–134.
Da Silva, V.E.L., and N.N. Fabre. 2019. Rare species enhance niche differentiation among tropical estuarine fish species. Estuaries and Coasts 42: 890–899.
Ditty, J.G., M. Bourgeois, R. Kasprzak, and M. Konikoff. 1991. Life history and ecology of sand seatrout Cynoscion arenarius Ginsburg, in the northern Gulf of Mexico: A review. Northeast Gulf Science 12 (1): 35–47.
Dormann, C.F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, J.R. García Marquéz, B. Gruber, B. Lafourcade, P.J. Leitão, T. Münkemüller, C. McClean, P.E. Osborne, B. Reineking, B. Schröder, A.K. Skidmore, D. Zurell, and S. Lautenbach. 2013. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36 (1): 27–46.
Drinkwater, K.F., and K.T. Frank. 1994. Effects of river regulation and diversion on marine fish and invertebrates. Aquatic Conservation: Freshwater and Marine Ecosystems 4: 135–151.
Duggan, Melissa, Peter Bayliss, and Michele A. Burford. 2019. "Predicting the impacts of freshwater-flow alterations on prawn (Penaeus merguiensis) catches." Fisheries Research 215: 27-37.
Erwin, K.L. 2009. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetlands Ecology and Management 17: 71–84.
Estevez, E.D. 2002. Review and assessment of biotic variables and analytical methods used in estuarine inflow studies. Estuaries 25 (6B): 1291–1303.
Faunce, C.H., J.E. Serafy, and J.J. Lorenz. 2004. Density-habitat relationships of mangrove creek fishes within the southeastern saline Everglades (USA), with reference to managed freshwater releases. Wetlands Ecology and Management 12: 377–394.
Gandy, D.A., and J.S. Rehage. 2017. Examining gradients in ecosystem novelty: Fish assemblage structure in an invaded Everglades canal system. Ecosphere 8 (1): e01634.
Gillanders, B.M., and M.J. Kingsford. 2002. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanography and Marine Biology, an Annual Review 40: 233–309.
Gillson, J. 2011. Freshwater flow and fisheries production in estuarine and coastal systems: Where a drop of rain is not lost. Reviews in Fisheries Science 19 (3): 168–186.
Gotelli, N.J., and R.K. Colwell. 2001. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Grange, N., A.K. Whitfield, C.J. De Villiers, and B.R. Allanson. 2000. The response of two South African east coast estuaries to altered river flow regimes. Aquatic Conservation: Marine and Freshwater Ecosystems 10: 155–177.
Greenwood, M.F.D., P.W. Stevens, and R.E. Matheson Jr. 2006. Effects of the 2004 hurricanes on the fish assemblages in two proximate southwest Florida estuaries: Change in the context of interannual variability. Estuaries and Coasts 29 (6A): 985–996.
Hooper, D.U., F.S. Chapin III., J.J. Ewel, A. Hector, P. Inchausti, S. Lavorel, J.H. Lawton, D.M. Lodge, M. Loreau, S. Naeem, B. Schmid, H. Setala, A.J. Symstad, J. Vandermeer, and D.A. Wardle. 2005. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecological Monographs 75 (1): 3–35.
Idelberger, C.F., and M.F.D. Greenwood. 2005. Seasonal variation in fish assemblages within the estuarine portions of the Myakka and Peace Rivers. Southwest Florida. Gulf of Mexico Science 2005 (2): 224–240.
Irlandi, E., S. Macia, and J. Serafy. 1997. Salinity reduction from freshwater canal discharge: Effects on mortality and feeding of an urchin (Lytechinus variegatus) and a gastropod (Lithopoma tectum). Bulletin of Marine Science 61 (3): 869–879.
Jenkins, G.P., S.D. Conron, and A.K. Morison. 2010. Highly variable recruitment in an estuarine fish is determined by salinity stratification and freshwater flow: Implications of a changing climate. Marine Ecology Progress Series 417: 249–261.
Jones, P.W., F.D. Martin, and J.D. Hardy Jr. 1978. Development of fishes of the Mid-Atlantic Bight. An atlas of eggs, larval and juvenile stages. Vol. 1. Acipenseridae through Ictaluridae. U.S. Fish and Wildlife Service Biol. Serv. Program.
Kanandjembo, A.N., I.C. Potter, and M.E. Platell. 2001. Abrupt seasonal shifts in the fish community of the hydrologically variable upper estuary of the Swan River. Hydrological Processes 15: 2503–2517.
Kendall, M.S., B.L. Williams, P.M. O’Donnell, B. Jessen, and J. Drevenkar. 2022. Too much freshwater, not enough, or just right? Long-term trawl monitoring demonstrates the impact of canals that altered freshwater flow to three bays in SW Florida. Estuaries & Coasts 45: 2710–2727.
Koenig, C.C., F.C. Coleman, A.M. Eklund, J. Schull, and J. Ueland. 2007. Mangroves as essential nursery habitat for goliath grouper (Epinephelus itajara). Bulletin of Marine Science 80 (3): 567–586.
Krauss, K.W., A.S. From, T.W. Doyle, T.J. Doyle, and M.J. Barry. 2011. Sea-level rise and landscape change influence mangrove enroachment onto marsh in the Ten Thousand Islands regions of Florida, USA. Journal of Coastal Conservation 15: 629–638.
Lee, T.N., N. Melo, N. Smith, E.M. Johns, C.R. Kelble, R.H. Smith, and P.B. Ortner. 2016. Circulation and water renewal of Florida Bay, USA. Bulletin of Marine Science 92 (2): 153–180.
Ley, J.A., C.L. Montague, and C.C. McIvor. 1994. Food habits of mangrove fishes: A comparison along estuarine gradients in northeastern Florida Bay. Bulletin of Marine Science 53 (3): 881–889.
Ley, J.A., C.C. McIvor, and C.L. Montague. 1999. Fishes in mangrove prop-root habitats of northeastern Florida Bay: Distinct assemblages across an estuarine gradient. Estuarine, Coastal and Shelf Science 48: 701–723.
Loh, A.N., L. Hermabessiere, P. Goodman, A.K. Volety, and P. Soudant. 2017. Impacts of altered hydrology on the sources of particulate organic carbon on the diet of Crassostrea virginica in the Northern Everglades, FL, USA. Journal of Shellfish Research 36 (3): 707–715.
Lorenz, J.J. 1999. The response of fishes to physiochemical changes in the mangroves of northeast Florida Bay. Estuaries 22 (2B): 500–517.
Lorenz, J.J., and J.E. Serafy. 2006. Subtropical wetland fish assemblages and changing salinity regimes: Implications for everglades restoration. Hydrobiologia 569: 401–422.
Lorenz, J.J. 2014. A review of the effects of altered hydrology and salinity on vertebrate fauna and their habitats in northeastern Florida Bay. Wetlands 34 (S1): 189–200.
Montague, C.L., and J.E. Ley. 1993. A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in northeastern Florida Bay. Estuaries 16: 703–717.
Mouillot, D., D.R. Bellwood, C. Baraloto, J. Chave, R. Galzin, M. Harmelin-Vivien, M. Kulbicki, S. Lavergne, S. Lavorel, N. Mouquet, C.E.T. Paine, J. Renaud, and W. Thuiller. 2013. Rare species support vulnerable functions in high-diversity ecosystems. Plos Biology 11 (5): e1001569.
NOAA NERRS. 2020. National Estuarine Research Reserve System, System-wide Monitoring Program. Data accessed from the NOAA NERRS Centralized Data Management Office website: http://www.nerrsdata.org. Accessed 14 Dec 2020.
Olin, J.A., P.W. Stevens, S.A. Rush, N.E. Hussey, and A.T. Fisk. 2015. Loss of seasonal variability in nekton community structure in a tidal river: Evidence for homogenization in a flow-altered system. Hydrobiologia 744: 271–286.
Palmer, T.A., P.A. Montagna, J.B. Pollack, R.D. Kalke, and H.R. DeYoe. 2011. The role of freshwater inflow in lagoons, rivers, and bays. Hydrobiologia 667: 49–67.
Palmer, T.A., P.A. Montagna, R.H. Chamberlain, P.H. Doering, Y. Wan, K.M. Haunert, and D.J. Crean. 2015. Determining the effects of freshwater inflow on benthic macrofauna in the Caloosahatchee Estuary. Florida Integrated Environmental Assessment and Management 12 (3): 529–539.
Patillo, M.E., T.E. Czapla, D.M. Nelson, and M.E. Monaco. 1997. Distribution and abundance of fishes and invertebrates in Gulf of Mexico estuaries. Volume II: Species life history summaries. ELMR Rep. No. 11. NOAA/NOS Strategic Environmental Assessments Division, Silver Spring, MD. 377 pp.
R Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rehage, J.S., R.E. Boucek, R.O. Santos, J.A. Massie, N.M. Viadero, and R.J. Rezek. 2022. Untangling flow-ecology relationships: Effects of seasonal stage variation on common snook aggregation and movement rates in the Everglades. Estuaries and Coasts 45: 2059–2069.
Reichert, M.J.M., and H.W. van der Veer. 1991. Settlement, abundance, growth and mortality of juvenile flatfish in a subtropical tidal estuary (Georgia, USA). Netherlands Journal of Sea Research 27 (3): 375–391.
Robins, J.B., I.A. Halliday, J. Staunton-Smith, D.G. Mayer, and M.J. Sellin. 2005. Freshwater-flow requirements of estuarine fishes in tropical Australia: A review of the state of knowledge and application of a suggested approach. Marine and Freshwater Research 56: 343–360.
Ross, S.T. 2001. Inland Fishes of Mississippi. University Press of Mississippi.
Rubec, P.J., C. Santi, X. Chen, and Y. Ghile. 2021. Habitat suitability modeling and mapping to assess the influence of freshwater withdrawals on spatial distributions and population numbers of estuarine species in the Lower Peace River and Charlotte Harbor, Florida. Marine and Coastal Fisheries 13: 31–58.
SEDAR. 2018. SEDAR 51 – Gulf of Mexico Gray Snapper Stock Assessment Report. SEDAR, North Charleston, SC. 248 pp.
Serafy, J.E., K.C. Lindeman, T.E. Hopkins, and J.S. Ault. 1997. Effects of freshwater canal discharge on fish assemblages in a subtropical bay: Field and laboratory observations. Marine Ecology Progress Series 160: 161–172.
Serrano, X., M. Grosell, and J.E. Serafy. 2010. Salinity selection and preference of the grey snapper Lutjanus griseus: Field and laboratory observations. Journal of Fish Biology 76: 1592–1608.
Sheaves, M. 1996. Do spatial differences in the abundance of two serranid fishes in estuaries of tropical Australia reflect long-term salinity patterns? Marine Ecology Progress Series 137: 39–49.
Shirley, M., P. O’Donnell, V. McGee, and T. Jones. 2004. Nekton species composition as a biological indicator of altered freshwater inflow: A comparison of three South Florida Estuaries. In Estuarine Indicators, ed. S. Bortone, 351–363. Boca Raton, FL: CRC Press.
Soderqvist, L.E., and E. Patino. 2010. Seasonal and spatial distribution of freshwater flow and salinity in the Ten Thousand Islands estuary, Florida, 2007-2009. U.S. Geological Survey Data Series 501: 24.
South Florida Ecosystem Restoration Task Force. 2020. South Florida Ecosystem Restoration Task Force: 2020 Biennial Report. U.S. Department of the Interior - Office of Everglades Restoration.
Stevens, P.W., M.F.D. Greenwood, C.F. Idelberger, and D.A. Blewett. 2010. Mainstem and backwater fish assemblages in the tidal Caloosahatchee River: Implications for freshwater inflow studies. Estuaries and Coasts 33: 1216–1224.
Stevens, P.W., M.F.D. Greenwood, and D.A. Blewett. 2013. Fish assemblages in the oligohaline stretch of a Southwest Florida river during periods of extreme freshwater inflow variation. Transactions of the American Fisheries Society 142 (6): 1644–1658.
Stevens, P.W., D.A. Blewett, R.E. Boucek, J.S. Rehage, B.L. Winner, J.M. Young, J.A. Whittington, and R. Paperno. 2016. Resilience of a tropical sport fish population to a severe cold event varies across five estuaries in southern Florida. Ecosphere 7 (8): e01400.
Terwilliger, M.R., and T.A. Munroe. 1999. Age, growth, longevity, and mortality of blackcheek tonguefish, Symphurus plagiusa (Cynoglossidae: Pleuronectiformes), in Chesapeake Bay, Virginia. Fishery Bulletin 97: 340–361.
Tolley, S.G., A.K. Volety, M. Savarese, L.D. Walls, C. Linardich, and E.M. Everham III. 2006. Impacts of salinity and freshwater inflow on oyster-reef communities in Southwest Florida. Aquatic Living Resources 19: 371–387.
U.S. Army Corps of Engineers. 2004. Picayune Strand Restoration Project (formerly Southern Golden Gate Estates Ecosystem Restoration): Final Integrated Project Implementation Report and Environmental Impact Statement, Jacksonville, Fla., 414 pp.
Van Niekerk, L., J.B. Adams, D.G. Allan, S. Taljaard, S.P. Weerts, D. Louw, C. Talanda, and P. Van Rooyen. 2019. Assessing and planning future estuarine resource use: A scenario-based regional-scale freshwater allocation approach. Science of the Total Environment 657: 1000–1013.
Vinagre, C., J. Salgado, H.N. Cabral, and M.J. Costa. 2011. Food web structure and habitat connectivity in fish estuarine nurseries – impact of river flow. Estuaries and Coasts 34: 663–674.
Walton, B.W., C.F. Cotton, D.A. Gandy, and M.M.P.B. Fuentes. 2022. Determining the influence of abiotic factors on spatial-temporal patterns of marine catfish (family: Ariidae) within the Apalachicola Bay System, Florida. Environmental Biology of Fishes 105: 369–383.
Whaley, S.D., M.C. Christman, and J.J. Burd Jr. 2016. Spatial distribution-abundance relationships in juvenile (age 0) red drum (Sciaenops ocellatus) and spotted seatrout (Cynoscion nebulosus). I: Influence of freshwater inflow. Estuaries and Coasts 39: 742–751.
Whitehead, P.J.P. 1988. FAO species catalogue: an annotated and illustrated catalogue of the Herrings, Sardines, Pilchards, Sprats, Shads, Anchovies and Wolf-Herrings (Vol. 7). Rome Italy: Food & Agriculture Org.
Wilson, J.K., A.J. Adams, and R.N.M. Ahrens. 2019. Atlantic tarpon (Megalops atlanticus) nursery habitats: Evaluation of habitat quality and broad-scale habitat identification. Environmental Biology of Fishes 102: 383–402.
Wingard, G.L., and J.J. Lorenz. 2014. Integrated conceptual ecological model and habitat indices for the southwest Florida coastal wetlands. Ecological Indicators 44: 92–107.
Wood, S.N. 2017. Generalized additive models: An introduction with R, 2nd ed. New York, NY: Chapman and Hall/CRC.
Yokel, B.J. 1975. A comparison of animal abundance and distribution in similar habitats in Rookery Bay, Marco Island and Fakahatchee on the southwest coast of Florida 1971–1972. University of Miami, Rosentiel School of Marine and Atmospheric Science. Preliminary report to the Deltona Corporation. Available as 2006 NOAA Technical Memorandum NOS NCCOS 35 and RSMAS TR 2006–03. Ed. A.Y. Cantillo.
Acknowledgements
This analysis was funded by NCCOS Project 848. The trawl program was funded by the Florida Department of Environmental Protection. The authors thank the thousands of volunteers who contributed countless hours assisting with data collection, especially the late Jean Barden who quality assured over a decade of trawl data. Water quality data was provided by Rookery Bay staff including Heather Stoffel, Vickie McGee, Christina Panko-Graff, and Julie Drevenkar. GIS base layers were provided by Jill Schmid. Dan Crear provided comments on the analytical framework. John Christensen and Christopher Jeffrey provided constructive review comments. Field sampling was conducted under FWC permit SAL-20-0059-SRP in consultation with the Ten Thousand Islands National Wildlife Refuge.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S. Peterson
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Williams, B.L., O’Donnell, P.M., Kendall, M.S. et al. How Are Man-Made Changes in Freshwater Flow Related to the Abundance of Juvenile Estuarine Fishes?. Estuaries and Coasts 46, 1632–1651 (2023). https://doi.org/10.1007/s12237-023-01232-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01232-8