Abstract
It is widely assumed that phytoplankton abundance and productivity decline during temperate winters because of low irradiance and temperatures. However, winter phytoplankton blooms commonly occur in temperate estuaries, but they are often undocumented because of reduced water quality monitoring in winter. The small body of in situ work that has been done on winter blooms suggests they can be of enormous consequence to ecosystems. However, because monitoring is often reduced or stopped altogether during winter, it is unclear how widespread these blooms are or how long they can last. We analyzed an over 30-year record of monthly phytoplankton monitoring samples along with ad hoc sampling throughout Chesapeake Bay to assess the distributions of two common winter bloom species, Heterocapsa rotundata and Heterocapsa steinii, and the environmental conditions associated with these blooms. The long-term monitoring data revealed that H. rotundata blooms occur within a narrow salinity range (7–12) and potentially have different triggers depending upon the nutrient status of waters affected. The ad hoc sampling confirmed the occurrence of H. steinii blooms in the lower Chesapeake Bay, despite the lack of evidence for them from monthly monitoring data. Together, our findings demonstrate that winter blooms routinely occur in numerous locations throughout Chesapeake Bay and can last up to a month. Our findings suggest that while winter blooms are a staple of the Bay’s annual phytoplankton community, there are major data gaps reporting their occurrence highlighting the need for more frequent monitoring to understand factors promoting these blooms and their consequences on ecosystem productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton form the base of most aquatic food webs and produce the majority of autochthonous carbon in aquatic ecosystems. Long-term datasets suggest that in temperate coastal and estuarine regions, such as the Chesapeake Bay, phytoplankton biomass and productivity tend to be highest in the spring and summer, respectively (Adolf et al. 2006; Marshall et al. 2009; Nesius et al. 2007). Because winter phytoplankton biomass and productivity are often low compared to the remainder of the year (Nesius et al. 2007), it is common for monitoring and research aimed at phytoplankton to be suspended during this period (December to February). However, although winter phytoplankton blooms are known to occur in temperate estuaries, they are under sampled due to the reduction in field sampling during winter. Heterocapsa rotundata and H. steinii (cf. Heterocapsa triquetra, Tillmann et al. 2017) are two dinoflagellate species that commonly bloom in winter (Cohen 1985; Sellner et al. 1991; Litaker et al. 2002a, b; Millette et al. 2015, 2017; Mulholland et al. 2018). Previous research suggests that winter blooms of these species can account for ~50% of the annual phytoplankton carbon production in the areas they occur, highlighting their potential to be an important contribution to annual productivity in estuarine systems (Sellner et al. 1991). Unfortunately, due to limited monitoring and phytoplankton research during winter, the coverage and duration of these blooms remain largely unknown. As a result, it is difficult to estimate how important winter blooms are to community production in estuarine ecosystems. If these blooms are highly localized and short-lived, then their ecosystem impact is likely to be minimal. However, if these blooms are a common occurrence throughout a substantial part of a system, then their ecosystem impact needs to be quantified.
If Heterocapsa blooms account for ~50% of the annual production, as suggested by Sellner et al. (1991), that means we currently have a limited understanding of the controls on total primary productivity and its cascading effects on ecosystems where winter blooms occur. For example, modeling work by Testa et al. (2018) suggests that winter blooms in the Chesapeake Bay are associated with the earlier onset of hypoxic conditions in summer. Alternatively, there is some evidence that winter H. rotundata blooms increase spring copepod populations, which are important prey for fish larvae (Millette et al. 2016, 2020). All this research points to potentially major ecosystem impacts of winter blooms, assuming they are widespread and long lasting in a system.
The majority of the work examining winter phytoplankton blooms to date has been conducted in Chesapeake Bay, USA. These studies have focused on understanding factors promoting blooms of H. rotundata in tributaries that flow into the mainstem of the Bay, e.g., the Potomac (Cohen 1985), Patuxent (Sellner et al. 1991), Choptank (Millette et al. 2015, 2017), and James Rivers (Mulholland et al. 2018), or quantifying the occurrence of H. rotundata and H. steinii in the lower Chesapeake Bay tributaries (Marshall and Egerton 2009; Mulholland et al. 2018). Based on studies in Maryland tributaries, it has been hypothesized that H. rotundata are more likely to form blooms in wet and cold winters (Cohen 1985; Millette et al. 2015). Winters with above average rain and snowfall result in increased freshwater fluxes into the Bay which likely increase the concentration of available nutrients during winter and spring and create a more stratified water column, both factors that favor H. rotundata over diatoms (Cohen 1985). It has been suggested that grazing by the copepod Eurytemora carolleeae provides a top-down control on H. rotundata blooms (Sellner et al. 1991). Winters with water temperatures below 1–2 °C also appear to release H. rotundata from zooplankton grazing pressure (Millette et al. 2015). While few studies have examined winter-time blooms of H. steinii, there are data suggesting that they can dominate the phytoplankton community in the lower Chesapeake Bay during winter (Marshall et al. 2005; Mulholland et al. 2018) and reports of winter H. steinii blooms in North Carolina estuaries during wet winters (Litaker et al. 2002a, b).
While these studies have provided insights into biotic and abiotic factors that may control winter blooms, they do not address how widespread these blooms are, nor do they describe the environmental niche occupied by either Heterocapsa spp. because sampling was limited to periods when there were active blooms and lacked spatial coverage. Here, we examined the Bay-wide distribution and variability of winter populations of H. rotundata and H. steinii with respect to environmental conditions in the Chesapeake Bay. We used Chesapeake Bay Monitoring Program data (1985–2020) and ad hoc data collected as part of specific projects to examine (1) the monthly mean abundances of H. rotundata and H. steinii across seasons throughout Chesapeake Bay, and (2) identify conditions that might favor high abundances of both species in the winter season. To grasp the extent of Heterocapsa spp. winter blooms and their impacts on estuarine ecosystems, we need to first assess where and why these blooms occur, the magnitude and duration of these blooms, and which Heterocapsa species dominate the blooms.
Methods
Chesapeake Bay Program Monitoring Data: Existing Data
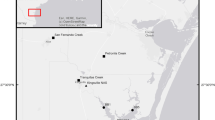
In Maryland, phytoplankton samples were collected once per month from a subset of the Chesapeake Bay Program Monitoring (CBPM) stations, three mainstem and five tributary stations, between 1985 and 2009 (Fig. 1). Similarly, phytoplankton samples were collected monthly from a subset of the CBPM stations in Virginia, seven mainstem and seven tributary stations, since 1985 (Fig. 2). At the mainstem stations, two 15-L composite samples were collected, one from above the pycnocline and the other from below the pycnocline. At the tributary stations, only a single 15-L composite sample was collected at each station. A portion of each composite sample (500 mL) was fixed with Lugol’s solution and preserved with buffered formalin for phytoplankton analysis. Phytoplankton samples were settled in Utermöhl chambers and enumerated using an inverted microscope. Samples were analyzed to the lowest practical taxonomic level, which, in connection to this study, was species level. In the Maryland and most of the Virginia dataset, H. rotundata is identified by its old name, Katodinium rotundatum. However, starting in 2014, the Virginia dataset transitioned to using the current name for H. rotundata.
Average seasonal abundances of H. rotundata (green) and H. steinii (blue) at eight different CBPM monitoring stations collected between 1986 and 2009 in the Maryland half of Chesapeake Bay. W = January–March, Sp = April–June, Sm = July–September, F = October–December. Error bars represent standard error
Average seasonal abundances of H. rotundata (green) and H. steinii (blue) at fourteen different CBPM monitoring stations collected between 1986 and 2019 in the Virginia half of Chesapeake Bay. W = January–March, Sp = April–June, Sm = July–September, F = October–December. Error bars represent standard error
At the same CBPM stations where samples were collected for phytoplankton enumeration, samples were also collected from above and below the pycnocline to measure dissolved inorganic nutrient concentrations and chlorophyll a. Nutrient, chlorophyll, and total suspended solids (TSS) samples were collected 1 m below the surface and above the bottom. Vertical profiles of hydrographic properties (temperature, salinity, etc.) were collected using a SeaBird CTD mounted to a rosette equipped with 10 Niskin bottles. Nutrient samples (nitrate + nitrite [NOx], ammonium, and phosphate) were filtered onboard, frozen, and then analyzed in the laboratory on a Lachat nutrient analyzer using standard colorimetric methods (USEPA 1993) according to the manufacturer’s specifications. Chlorophyll a concentrations were measured spectrophotometrically (Strickland and Parsons 1972; APHA 1995). TSS were measured after drying (APHA 1989).
Ad Hoc Data: New Data
Weekly Grab Samples–Choptank River, MD
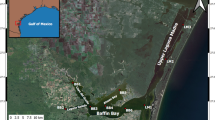
Samples were collected at least once a week from 2012 to 2016 at the Bill Burton fishing pier on the Choptank River in Cambridge, MD, USA (38°34′24″ N 76°4′6″ W), a tributary that feeds into the mesohaline section of the Chesapeake Bay (Fig. 1). H. rotundata abundance was recorded once a week in 2012 (January, 23 2012–March, 13 2012), 2013 (December 23, 2012–March 10, 2013), 2014 (December 30, 2013–April 14, 2014), and 2015 (December 29, 2014–March 9, 2015) and three times a week in 2016 (December 30, 2015–March 18, 2016). For some years, abundance data for additional phytoplankton species were collected (Millette et al. 2015), but only H. rotundata abundance is presented. The fishing pier is located near the CBPM station ET5.2 (<0.5 km away), which was included in our analysis of CBPM data.
Water from the surface was collected using a bucket and 15 mL was preserved in scintillation vials with 5% acid Lugol’s. Triplicate samples were preserved and counted for each sampling date. Heterocapsa rotundata were identified and enumerated with a Nikon Eclipse E800 microscope at 20× magnification on a Sedgewick rafter slide (Sherr and Sherr 1993). A minimum of 300 cells were counted per sample.
Imaging FlowCytoBot Data–Elizabeth River, VA
Samples were collected 4–5 times per week from mid-January to late March 2020 at the Old Dominion University (ODU) Sailing Center floating dock on the Elizabeth River in Norfolk, VA, USA (36°52′10″ N, 76°19′4″ W), a sub-tributary of the polyhaline section of the Chesapeake Bay. Whole water was collected from the surface with a 500-mL amber polycarbonate sample bottle and taken directly back to the laboratory at ODU (~10 min walk) to be processed using an Imaging Flow CytoBot imaging flow cytometer (IFCB; Olson and Sosik 2007). Samples were run in triplicate for each time point. The resulting images were processed using the MATLAB ifcb-analysis toolbox (https://github.com/hsosik/ifcb-analysis) and then uploaded to the EcoTaxa web application (https://ecotaxa.obs-vlfr.fr/; Picheral et al. 2020) for taxonomic identification, annotation, and automated classification of images. The classified images were used in combination with the data on the volume imaged by the IFCB to derive taxa-specific abundances for each sample.
CBPM Data Synthesis and Analysis
We averaged the abundances of H. rotundata and H. steinii at each station for each season; winter (Jan.–Mar.), spring (Apr.–Jun.), summer (Jul.–Sept.), and fall (Oct.–Dec.), in order to look at temporal and spatial variability in Heterocapsa spp. within the large CBPM program dataset. Next, we analyzed environmental conditions associated with the upper (25%) versus lower quartile (75%) abundance of Heterocapsa spp. in winter (Jan.–Mar.) at stations where H. rotundata or H. steinii had higher abundances during the winter compared to other seasons (see Figs. 1 and 2). We compared the average values for all environmental factors associated with stations in the upper quartile for Heterocapsa spp. abundance and compared these to observations made for the lower quartile using a t-test with equal variance. We chose to use upper and lower quartiles of abundances for our analysis because assigning a specific concentration at which Heterocapsa spp. has formed a bloom is arbitrary and likely varies spatially for both species. If there was a significant difference between chlorophyll a concentrations for the upper and lower quartiles associated with the increase in Heterocapsa spp. abundance, then it is more likely that the shift in abundance was the result of a bloom and not a shift in phytoplankton community composition.
Results
Analysis of CBPM Data
Spatial Variability in Heterocapsa Blooms
Based on the CBPM data, average abundances of Heterocapsa spp. were an order of magnitude higher in the Maryland portion of the Bay compared to Virginia (Figs. 1 and 2). In the Maryland CBPM data, it is clear that H. rotundata abundances are highest during the winter season at certain stations but H. steinii abundances do not appear to vary across seasons (Fig. 1). The average winter abundance of H. rotundata was highest at stations in the upper regions of the mainstem (CB3.3C) and three upper Bay tributaries (ET5.2, LE1.1, and WT5.1). In the Virginia portion of the Bay, H. rotundata or H. steinii abundances rarely differed between seasons; however, evidence suggests that blooms occur there with some frequency. This evidence includes (1) high average abundances of H. rotundata at station RET3.1 in winter and H. steinii at station RET4.3 in winter and spring (Fig. 2), and (2) ad hoc bloom sampling (Marshall et al. 2005, 2009; Mulholland et al. 2018). When looking at the Chesapeake Bay as a whole, Heterocapsa spp. are rare at tidal fresh (TF) stations and H. rotundata abundance appears to follow a gradient along the Bay’s mainstem, with higher abundances in the less saline upper Bay and lower abundances in the lower Bay (Figs. 1 and 2).
Realized Temperature and Salinity Niche Space
The realized niches of H. rotundata and H. steinii in temperature and salinity space were inferred separately for the MD (Fig. 3) and VA (Fig. 4) datasets. In MD, the highest mean abundances of H. rotundata were associated with water temperatures ranging from 0 to 10 °C, and salinities between 7 to 12. In contrast, the highest abundances of H. steinii were observed in warmer waters, with the highest abundances observed when water temperatures were 13–19 °C. H. steinii also occurred over a wider salinity range, salinities ranging from 5 to 21, suggesting a less restricted salinity niche space than that of H. rotundata.
Mean abundances of Heterocapsa rotundata (cells mL−1; left panel) and Heterocapsa steinii (cells mL−1; right panel) in the MD half of the Chesapeake Bay for the full range of temperature and salinity conditions observed over the course of the time series. The shaded gray area denotes parts of the variable space where no data was present
Mean abundances of Heterocapsa rotundata (cells mL−1; left panel) and Heterocapsa steinii (cells mL−1; right panel) in the VA half of the Chesapeake Bay for the full range of temperature and salinity conditions observed over the course of the time series. The shaded gray area denotes parts of the variable space where no data was present
In Virginia, H. rotundata appeared to occupy somewhat different temperature and salinity niches than in Maryland. High mean abundances of H. rotundata were found over a wider and warmer range of water temperature (10 to 27 °C) and salinity (5–30) in Virginia, although their mean abundance tended to decrease at higher salinities. While both are eurythermal and euryhaline, H. steinii appears to have a narrower temperature and salinity range than H. rotundata. The highest mean abundances of H. steinii were found when water temperatures were between 10 and 18 °C, and although present at high salinities, most of the samples with mean cell abundances over 100 cells mL1 were collected from waters where salinities were between 7 and 20.
Bloom Conditions
We analyzed the relationship between environmental conditions and H. rotundata abundance (upper and lower quartiles of abundance) at 4 stations in Maryland where high winter abundances were routinely observed in Maryland (CB3.3C, ET5.2, LE1.1, and WT5.1). High concentrations of H. steinii and Heterocapsa spp. were not observed in the CBPM data from Maryland and Virginia, respectively (Figs. 1 and 2). Average H. rotundata abundances associated with the upper quartile at all 4 stations were an order of magnitude higher than average H. rotundata abundances associated with the lower quartile (Table 1). Furthermore, chlorophyll a concentrations were also significantly higher for the upper quartile of H. rotundata abundance compared to the lower quartile at all but one of the stations (WT5.1). At station CB3.3C, oxygen concentrations were significantly lower and total suspended solids were significantly higher for samples in the upper quartile of H. rotundata abundance than those in the lower quartile (Table 1). At station ET5.2, ammonium and Secchi depth were significantly lower for samples in the upper quartile of H. rotundata abundance than those in the lower quartile (Table 1). At station LE1.1, salinity was significantly lower and NOx concentrations and total suspended solids were significantly higher for samples in the upper quartile of H. rotundata abundances than those in the lower quartile (Table 1). At station WT5.1, dissolved oxygen was significantly lower for samples in the upper quartile of H. rotundata abundance than those in the lower quartile (Table 1).
Analysis of Ad Hoc Data
Data collected in the Choptank River, near station ET5.2, between 2012 and 2016 and data collected in the Lafayette River in 2020 provided a more detailed look at inter- and intra-annual variability (Fig. 5).
Short-term time series data for Heterocapsa spp. abundances in the a Choptank River, MD for five winters (2012–2016) and the b Lafayette River, VA for one winter (2020). 2013 and 2014 H. rotundata abundance data in the Choptank River was adapted from Millette et al. (2015)
Choptank River, MD
Winter blooms in the Choptank River were always dominated by H. rotundata; H. steinii typically accounted for less than 5% of the total abundance (Millette et al. 2015). The average abundance for H. rotundata was typically between 100 and 1000 cells mL−1 (Fig. 5a). Cell abundances reached at least 10,000 cells mL−1 in three of the winters (2014, 2015, and 2016), with abundances up to 32,000 cells mL−1 in 2014. Conversely, there was one year (2012) when cell abundances were substantially below average. In years when H. rotundata abundance reached at least 10,000 cells mL−1, cell abundances increased over a 4–5-week period before reaching their maximum abundances. Once maximum cell abundances were attained, usually around the end of February, blooms could persist for over a month (Fig. 5a).
Elizabeth River, VA
There was a winter bloom of H. steinii in the Elizabeth River in 2020. For most of the winter, H. steinii abundances were below 100 cells mL−1 but their abundances increased by up to two orders of magnitude during most of February. Unlike H. rotundata blooms in the Choptank River, this bloom appeared to initiate rapidly and then persisted for about 3 weeks (Fig. 5b). While abundances of H. rotundata also increased during this bloom (> 1000 cells mL−1), populations were dominated by H. steinii until early March when sampling was suspended.
Discussion
Using monthly samples collected as part of the CBPM over three decades along with more localized ad hoc sampling, we have established that winter Heterocapsa spp. blooms are a recurring feature within specific regions of Chesapeake Bay. Specifically, our analyses indicate that H. rotundata blooms are constrained to a tight salinity range (7–12) and predominate in the Maryland portion of the Bay. More importantly, despite the spatial constraint of these blooms due to salinity, the blooms can last for at least 1 month. Significantly higher abundances of H. rotundata in CBPM samples were associated with significantly higher chlorophyll a concentration (Table 1), increasing the likelihood that these abundances are associated with a bloom rather than a shift in phytoplankton community composition. While CBPM data provides no indication that H. steinii blooms occur in Chesapeake Bay, previous studies (Mulholland et al. 2018) and non-CBPM samples collected in Virginia indicate that H. steinii is the species that likely dominates winter blooms in the Virginia half of Chesapeake Bay. However, winter blooms in Virginia are undersampled, which limits our ability to say anything conclusive about these blooms.
Environmental Conditions Associated with H. rotundata Blooms
Taken as a whole, H. rotundata blooms in Maryland, and potentially all of the Bay, are tightly constrained by salinity (Fig. 4a). Our dataset-wide analysis of MD H. rotundata abundance demonstrated that the highest abundances occurred between salinities of 7 and 12. Furthermore, when comparing the average environmental conditions between all the Maryland stations, the only common factor between the four stations where H. rotundata blooms appeared to occur (CB3.3C, ET5.2, LE1.1, and WT5.1) was their salinity range (Table S1), which averaged between 9.6 and 12. Salinity was either significantly higher or lower than this range at the non-bloom stations. Previous research has suggested that H. rotundata blooms rarely occur above a salinity of 14 (Cohen 1985; Sellner et al. 1991). Our analysis supports this and further suggests that blooms rarely occur below a salinity of 7. This tight salinity range where MD H. rotundata blooms are found provides a clear expectation of where in Chesapeake Bay these blooms are likely to occur. However, it does not provide any information on other environmental conditions associated with these blooms.
Analysis of the four individual stations with H. rotundata blooms provided a more in-depth look at spatial variability in environmental conditions associated with these blooms and potential insight into conditions that favor the occurrence of winter blooms. Station ET5.2 (Choptank River) is located near where a series of studies were conducted on H. rotundata blooms from 2012 to 2016 (Millette et al. 2015, 2017; Millette 2016). Between 1985 and 2009, at the ET5.2 CBPM station, conditions associated with the upper quartile of H. rotundata abundance could be explained by conclusions from this previous research; specifically, there were lower ammonium concentrations, Secchi depths, and possibly temperature (Table 1). H. rotundata’s preferential consumption of ammonium (Millette 2016) could be the reason why ammonium concentrations were depleted during blooms. H. rotundata uses mixotrophy to compensate for light limitation of photosynthesis with heterotrophy (Millette et al. 2017), which can give them an advantage when irradiance levels are lower (low Secchi depths). However, neither of these factors provide any indication of how a bloom forms at ET5.2. In the CBPM monitoring dataset, differences in water temperature were not significant at station ET5.2, although the averages were still noticeably lower for the upper quartile H. rotundata abundances. Given more recent evidence that lower water temperatures provide a mechanism for relieving H. rotundata from top-down control in the Choptank River (Millette et al. 2015), it is likely that blooms at this station could have been associated with a decrease in temperature, which in turn decreased grazing pressure, rather than an increase in nutrients.
Alternatively, H. rotundata blooms at LE1.1 (Patuxent River) seem to be caused by an increase in freshwater flow, likely caused by rainfall. Station LE1.1 had lower average NOx and ammonium concentrations compared to most other stations, suggesting that the phytoplankton could be nutrient limited there (Table S1). The upper quartile of H. rotundata abundances at this station were associated with low salinity, high NOx concentrations, and no difference in ammonium concentrations (Table 1). This would suggest that H. rotundata at this station form blooms when an increase of freshwater input delivers new nutrients into the system, as evidenced by the decrease in salinity and increase in NOx concentrations. This is supported by research done on H. rotundata blooms in the Potomac River that came to similar conclusions showing that in years with higher rainfall, blooms formed (Cohen 1985). We propose that the lack of a difference in ammonium concentrations due to rainfall resulted from preferential uptake by H. rotundata.
Station CB3.3 (Upper Chesapeake Bay) lacked any clear indication to what environmental conditions might be associated with blooms at this location. The only significant difference between the upper quartile abundances of H. rotundata and lower abundances were lower dissolved oxygen concentrations and higher total suspended solids (Table 1). Oxygen concentrations are generally expected to be higher as a result of higher primary productivity and the high total suspended solids indicate that these blooms might have been light limited. H. rotundata can overcome light limitation of photosynthetic carbon fixation through mixotrophy (Millette et al. 2017). Lower dissolved oxygen concentrations could indicate that the blooms were dying off and starting to be respired. It is worth noting that while the difference was not significant, ammonium concentrations were noticeably lower when H. rotundata abundances were higher adding further support to observations that H. rotundata are preferentially removing ammonium from the water column.
Like station CB3.3C, upper quartile abundances of H. rotundata at station WT5.1 (Patapsco River) were associated with lower dissolved oxygen concentrations (Table 1). While ammonium concentrations were also noticeably lower when H. rotundata abundances were higher, the difference was not significant due to the high variability in concentrations (Table 1). This again suggests that H. rotundata is preferentially consuming ammonium over nitrate. Baltimore Harbor is located on the Patapsco River, and this large urban area is thought to contribute to the high nutrient concentrations and low water clarity observed there (Table S1). This would suggest that H. rotundata is not nutrient limited at this station; however, it could be light limited. There was no indication that water temperature had an influence on the blooms at this station as water temperatures were uniformly low whether H. rotundata was abundant or not. Although mean chl a concentrations were higher when H. rotundata was more abundant at WT5.1, unlike the other stations, the difference was not significant. Chl a concentrations were higher even when H. rotundata abundances were in the lower quartile suggesting that other species were abundant during the winter as well.
Based on our analysis of CBPM data and previous findings, it appears that there are at least two different conditions favoring the formation of winter H. rotundata blooms in the Maryland half of Chesapeake Bay. At a station like LE1.1, where nutrient concentrations are generally lower, an increase in freshwater input could trigger a winter H. rotundata bloom. When winter precipitation is high, new inorganic nitrogen is introduced into the system and a bloom forms (Cohen 1985). High precipitation would also result in low salinity and increased stratification, conditions that would favor dinoflagellates over diatoms (Cohen 1985). At a station like ET5.2, where nutrient concentrations are generally higher, but water clarity is low, blooms are more likely to be limited by light. Since H. rotundata blooms are able to overcome light limitation by employing mixotrophy (Millette et al. 2017), top-down control by microzooplankton and copepod grazers could be controlling the formation of blooms. When temperatures get low enough to limit grazing activity, phytoplankton are released from grazing pressure and can bloom (Millette et al. 2015). However, H. rotundata blooms will only occur under either of these conditions if they occur within the appropriate, narrow salinity range.
Despite the lack of data on H. steinii blooms in CBPM data, our ad hoc data suggests that H. steinii dominates winter blooms in the southern half of Chesapeake, and H. rotundata dominates in the northern half. We propose that the differences in the distributions of H. rotundata and H. steinii are primarily due to temperature. While our analysis of H. rotundata using CBPM data demonstrated a clear salinity preference, these salinities can be found in the southern half of the Bay (Tables S1 and S2), albeit at only a few stations, suggesting another factor influences this niche separation. Chesapeake Bay is elongated in the north–south direction and the more northern regions of the Bay experience colder average temperatures during the winter compared to the more southern regions (Tables S1 and S2). This makes temperature the most likely factor differentiating where H. rotundata and H. steinii dominate. Furthermore, winter blooms of H. steinii often occur in systems with winter temperatures above 5 °C (Litaker et al. 2002a; Montero et al. 2017). However, there is at least one recorded instance of H. steinii blooms under ice (Baek et al. 2011) and a lack of observed H. rotundata blooms at temperatures below 5 °C, other than in Chesapeake Bay. Temperature as the factor controlling niche separation of the two Heterocapsa spp. in Chesapeake Bay needs to be further explored, likely using a combination of laboratory and field measurements.
Undersampling of Heterocapsa Blooms–the Importance of High Frequency Monitoring
In Maryland, high frequency sampling was undertaken in a tidal tributary over 5 years (Millette 2016). These data provide insights regarding the timing and duration of H. rotundata blooms. While there was 1 year with high H. rotundata abundance during January, most blooms form throughout February and could last for at least a month once they reach their peak. The CBPM reliably collected one sample per year in mid- or late March, which means they were likely often sampling during the end of winter blooms. The higher frequency sampling makes it clear that H. rotundata blooms really do form in this system, and that these blooms can persist for a long time, likely contributing significantly to new production in the system. Previous research suggests that production from these blooms is likely consumed by zooplankton, benefiting higher trophic levels (Sellner et al. 1991; Millette et al. 2015, 2016, 2020). Unfortunately, because CBPM sampling in MD routinely stopped sampling during winter, there is almost no data on H. rotundata during the formation of their blooms which likely occurs before March.
While CBPM monitoring stations in Virginia had almost no indication that average Heterocapsa spp. abundance was higher in winter compared to other seasons (Fig. 2), previously published data (Marshall et al. 2005; Mulholland et al. 2018) and high frequency sampling in winter 2020 (Fig. 5) suggest blooms of this genera routinely occur in Virginia but are not being captured in the CBPM dataset. This may be due to temporal or spatial mismatches between Bay Program sampling and blooms, and highlights the need for better surveillance of phytoplankton populations in winter in Virginia as well as Maryland.
The Chesapeake Bay Program Monitoring provides valuable data for long-term analyses of phytoplankton community structure, diversity, and its change over time from fixed station monitoring. However, it was designed to monitor the long-term health of the Bay and does not have the resources to capture ephemeral or spatially confined blooms. Phytoplankton blooms are undersampled by the CBPM monitoring program in general, but during winter, undersampling is particularly acute as weather often curtails sampling. In Virginia, CBPM data suggests there are no winter blooms of Heterocapsa spp. in the southern half of Chesapeake Bay, despite additional data collected at other stations and with higher frequency suggesting otherwise (Fig. 5; Mulholland et al. 2018). In Maryland, CBPM data captured some of the H. rotundata blooms prior to 2009 but Maryland discontinued its phytoplankton monitoring program after 2009 and so even the most basic trend analysis, such as those employed here, is impossible because there is no data outside of ad hoc projects which are few and far between. Based on emerging issues and seasonal undersampling, the CBPM program data needs to be augmented with higher frequency time series sampling. Such higher frequency, winter sampling could be achieved with the deployment of one, or several, IFCBs at a small number of stations within the Bay. IFCBs can be deployed in the field for several months at a time and can transmit data back to shore in near real time. As such, deployments would allow for continuous remote monitoring over the winter and could also be used as a guide to trigger more targeted traditional sampling efforts if a bloom were observed to be initiating. The deployment of newer technologies such as IFCBs, in conjunction with more traditional sampling methods, would allow us to better understand the controls on winter bloom formation and decline, and aid in predicting how the occurrence of these blooms might change in the future.
Conclusions
Our analysis definitively demonstrates that (1) winter Heterocapsa spp. blooms are a common occurrence across Chesapeake Bay and its tributaries and (2) these blooms have been, and continue to be, chronically unsampled across large portions of the Bay. These blooms are a clear staple of the annual cycle within the Bay but outside of a few targeted studies, much about these blooms still remains unknown. Using long-term CBPM data, we were able to demonstrate that H. rotundata blooms occur in a tight salinity range (7–12) and propose that winter H. rotundata blooms form under at least two different conditions depending upon whether the blooms occur in a nutrient replete or nutrient limited environment. However, there is a lot more to study about these blooms, especially with their connection to higher trophic levels and influence on ecological processes during other seasons. For example, it has been hypothesized that E. carolleeae, a predator of H. rotundata, hatched in winter feed striped bass larvae hatched in spring (Millette et al. 2020) and high phytoplankton biomass earlier in the year will cause higher volumes of hypoxic waters to occur earlier in the summer (Testa et al. 2018).
While the research presented here has focused on these winter blooms in Chesapeake Bay, this is not the only system where Heterocapsa spp. occur. Heterocapsa spp. have been reported in a range of environments all over the world including Masan Bay, South Korea (Seong et al. 2006), Manori Creek and Manim Bay, India (Shahi et al. 2015), Baltic Sea, Germany (Jaschinski et al. 2015), Puyuhuapi Fjord, Chile (Montero et al. 2017), Sundays Estuary, South Africa (Lemley et al. 2018), Newport River, North Carolina (Litaker et al. 2002a,b), Johor Strait, Malaysia (Razali et al. 2022), and Kangaroo Island, Australia (Balzano et al. 2015). Wherever it is found, Heterocapsa spp. tend to either dominate or be a prominent part of the phytoplankton community, although not always prevalent when water temperatures are at their lowest (Seong et al. 2006; Balzano et al. 2015; Shahi et al. 2015). Given the widespread occurrence of Heterocapsa spp. in estuarine and coastal systems, their ability to be a prominent phytoplankton species, and their ability to form blooms during winter months, more research needs to focus on this genus. Future research targeted at observing winter Heterocapsa spp. blooms will expand our understanding of the full impact of these blooms on regional productivity, estuarine food webs and commercially important fisheries.
Data Availability
The majority of the data presented here was downloaded from the Chesapeake Bay Project Datahub, a publicly available dataset of plankton and water quality data for the Bay. Additional data for the Choptank River and Elizabeth River are available through the dissertation "Ecosystem Impact of Winter Dinoflagellate Blooms in the Choptank River, MD" in the Digital Repository at the University of Maryland and the Ecotaxa repository, respectively.
References
Adolf, J.E., C.L. Yeager, W.D. Miller, M.E. Mallonee, and L.W. Harding Jr. 2006. Environmental forcing of phytoplankton floral composition, biomass, and primary productivity in Chesapeake Bay, USA. Estuarine, Coastal and Shelf Science 67: 108–122. https://doi.org/10.1016/j.ecss.2005.11.030.
APHA. 1989. Method: 2540 D. Total suspended solids dried at 103-105oC. In Standard methods for the examination of water and wastewater, 17th ed. Washington, D.C.: American Public Health Association, American Water Works Association, and Water Pollution Control Federation.
APHA. 1995. Method: 10200 H. Chlorophyll. Spectrophotometric. In Standard methods for the examination of water and wastewater, 19th ed. Washington, D.C.: American Public Health Association, American Water Works Association, and Water Pollution Control Federation.
Baek, S.H., J.S. Ki, T. Katano, K. You, B.S. Park, H.H. Shin, K. Shin, Y.O. Kim, and M.-O. Han. 2011. Dense winter bloom of the dinoflagellate Heterocapsa triquetra below the thick surface ice of brackish Lake Shihwa, Korea. Phycological Research 59: 273–285. https://doi.org/10.1111/j.1440-1835.2011.00626.x.
Balzano, S., A.V. Ellis, C. Le Lan, and S.C. Leterme. 2015. Seasonal changes in phytoplankton on the north-eastern shelf of Kangaroo Island (South Australia) in 2012 and 2013. Oceanologia 57: 251–262. https://doi.org/10.1016/j.oceano.2015.04.003.
Cohen, R.R.H. 1985. Physical processes and the ecology of a winter dinoflagellate bloom of Katodinium rotundatum. Marine Ecology Progress Series 26: 135–144.
Jaschinski, S., S. Flöder, T. Petenati, and G. Göbel. 2015. Effects of nitrogen concentration on the taxonomic and functional structure of phytoplankton communities in the Western Baltic Sea and implications for the European water framework directive. Hydrobiologia 745: 201–210. https://doi.org/10.1007/s10750-014-2109-9.
Lemley, D.A., J.B. Adams, and G.M. Rishworth. 2018. Unwinding a tangle web: a fine-scale approach towards understanding the drivers of harmful algal bloom species in a eutrophic South African estuary. Estuaries and Coasts 41: 1356–1369. https://doi.org/10.1007/s12237-018-0380-0.
Litaker, R.W., P.A. Tester, C.S. Duke, B.E. Kenney, J.L. Pinckney, and J. Ramus. 2002a. Seasonal niche strategy of the bloom-forming dinoflagellate Heterocapsa triquetra. Marine Ecology Progress Series 232: 45–62. https://doi.org/10.3354/meps232045.
Litaker, R.W., V.E. Warner, C. Rhyne, C.S. Duke, B.E. Kenney, J. Ramus, and P.A. Tester. 2002b. Effect of diel and interday variations in light on the cell division pattern and in situ growth rates of the bloom-forming dinoflagellate Heterocapsa triquetra. Marine Ecology Progress Series 232: 63–74. https://doi.org/10.3354/meps232063.
Marshall, H.G., and T.A. Egerton. 2009. Phytoplankton blooms: their occurrence and composition within Virginia’s tidal tributaries. Virginia Journal of Science 60: 149–164. https://doi.org/10.25778/3kcs-7j11.
Marshall, H.G., L. Burchardt, and R. Lacouture. 2005. A review of phytoplankton composition within Chesapeake Bay and its tidal estuaries. Journal of Plankton Research 27: 1083–1102. https://doi.org/10.1093/plankt/fbi079.
Marshall, H.G., M.F. Lane, K.K. Nesius, and L. Burchardt. 2009. Assessment of significance of phytoplankton species composition within Chesapeake Bay and Virginia tributaries through a long-term monitoring program. Environmental Monitoring and Assessment 150: 143–155. https://doi.org/10.1007/s10661-008-0680-0.
Millette, N.C. 2016. Ecosystem impact of winter dinoflagellate blooms in the Choptank River. DRUM. https://doi.org/10.13016/M2VR81. Doctoral Dissertation.
Millette, N.C., J.J. Pierson, and D.K. Stoecker. 2015. Top-down control of micro-and mesozooplankton on winter dinoflagellate blooms of Heterocapsa rotundata. Aquatic Microbial Ecology 76: 15–25. https://doi.org/10.3354/ame01763.
Millette, N.C., G.E. King, and J.J. Pierson. 2016. A note on the survival and feeding of copepod nauplii (Eurytemora carolleeae) on the dinoflagellate Heterocapsa rotundata. Journal of Plankton Research 37: 1095–1099. https://doi.org/10.1093/plankt/fbv090.
Millette, N.C., J.J. Pierson, A. Aceves, and D.K. Stoecker. 2017. Mixotrophy in Heterocapsa rotundata: a mechanism for dominating the winter phytoplankton. Limnology and Oceanography 62: 836–845. https://doi.org/10.1002/lno.10470.
Millette, N.C., J.J. Pierson, and E.W. North. 2020. Water temperature during winter may control striped bass recruitment during spring by affecting the development time of copepod nauplii. ICES Journal of Marine Science 77: 300–314. https://doi.org/10.1093/icesjms/fsz203.
Montero, P., I. Pérez-Santos, G. Daneri, M.H. Gutiérrez, G. Igor, R. Seguel, D. Purdie, and D.W. Crawford. 2017. A winter dinoflagellate bloom drives high rates of primary production in a Patagonian fjord ecosystem. Estuarine, Coastal and Shelf Science 199: 105–116. https://doi.org/10.1016/j.ecss.2017.09.027.
Mulholland, M.R., R. Morse, T. Egerton, P.W. Bernhardt, and K.C. Filippino. 2018. Blooms of dinoflagellate mixotrophs in a lower Chesapeake Bay tributary: carbon and nitrogen uptake over diurnal, seasonal, and interannual timescales. Estuaries and Coasts 41: 1744–1765. https://doi.org/10.1007/s12237-018-0388-5.
Nesius, K.K., H.G. Marshall, and T.A. Egerton. 2007. Phytoplankton productivity in the tidal regions of four Chesapeake Bay (U.S.A) tributaries. Virginia Journal of Science 58: 194–204. https://digitalcommons.odu.edu/biology_fac_pubs/93. Accessed 24 October 2022.
Olson, R.J., and H.M. Sosik. 2007. A submersible imaging-in-flow instrument to analyze nano- and microplankton: Imaging FlowCytobot. Limnology and Oceanography: Methods 5: 95–203. https://doi.org/10.4319/lom.2007.5.195.
Picheral, M., S. Colin, and J.-O. Irisson. 2020. EcoTaxa, a tool for the taxonomic classification of images. http://ecotaxa.obs-vlfr.fr. Accessed 24 October 2022.
Razali, R.M., N.I. Mustapa, K.K.K. Yaacob, F. Yusof, S.T. Teng, A.H. Hanafiah, K.S. Hii, M. Mohd-Din, H. Gu, C.P. Leaw, and P.T. Lim. 2022. Diversity of Heterocapsa (Dinophyceae) and the algae bloom event in the mariculture areas of Johor Strait, Malaysia. Plankton and Benthos Research 17: 290–300. https://doi.org/10.3800/pbr.17.2.
Sellner, K.G., R.V. Lacouture, S.J. Cibik, A. Brindley, and S.G. Brownlee. 1991. Importance of a winter dinoflagellate-microflagellate bloom in the Patuxent River estuary. Estuarine, Coastal and Shelf Science 32: 27–42. https://doi.org/10.1016/0272-7714(91)90026-8.
Seong, K.A., H.J. Jeong, S. Kim, G.H. Kim, and J.H. Kang. 2006. Bacterivory by co-occurring red-tide algae, heterotrophic nanoflagellates, and ciliates. Marine Ecology Progress Series 322: 85–97. https://doi.org/10.3354/meps322085.
Shahi, N., A. Godhe, S.K. Mallik, K. Härnstrm, and B.B. Nayak. 2015. The relationship between variation of phytoplankton species composition and physico-chemical parameters in northern coastal waters of Mumbai, India. Indian Journal of Geo-Marine Sciences 44. http://nopr.niscpr.res.in/handle/123456789/34792. Accessed 24 October 2022.
Sherr, E. B., and Sherr, B. F. 1993. Preservation and storage of samples for enumeration of heterotrophic protists. Kemp, P. F., Sherr, B. F., Sherr, E. B., and Cole, J. J. (Eds.), Handbook of Methods in Aquatic Microbial Ecology, Lewis Publishers, Boca Raton, pp, 207–212.
Strickland, J.D.H., and T.R. Parsons. 1972. A practical handbook of seawater analysis. Ottawa, Canada: Bulletin Fisheries of the Research Board of Canada.
Testa, J.M., R.R. Murphy, D.C. Brady, and W.M. Kemp. 2018. Nutrient- and climate-induced shifts in the phenology of linked biogeochemical cycles in a temperate estuary. Frontiers in Marine Science 5: 114. https://doi.org/10.3389/fmars.2018.00114.
Tillmann, U., M. Hoppenrath, M. Gottschling, W.H. Kusber, and M. Elbrächter. 2017. Plate pattern clarification of the marine dinophyte Heterocapsa triquetra sensu Stein (Dinophyceae) collected at the Kiel Fjord (Germany). Journal of Phycology 53: 1305–1324. https://doi.org/10.1111/jpy.12584.
USEPA. 1993. Methods for chemical analysis of water and wastes. Cincinnati, Ohio: United States Environmental Protection Agency Publication Number EPA-600/4-79-020.
Funding
SC was supported by a Program for Undergraduate Research and Scholarship (PURS) grant from the Office of Research and Perry Honors College at Old Dominion University, Norfolk, VA, United States. MRM was supported by contracts from the Virginia Department of Environmental Quality, the Virginia Department of Health and the Hampton Roads Sanitation District.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hongbin Liu
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Millette, N.C., Clayton, S., Mulholland, M.R. et al. The Importance of Winter Dinoflagellate Blooms in Chesapeake Bay—a Missing Link in Bay Productivity. Estuaries and Coasts 46, 986–997 (2023). https://doi.org/10.1007/s12237-023-01191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01191-0