Abstract
The major nesting sites for the endangered green turtle (Chelonia mydas) are in Campeche, Mexico: Carmen, Isla Aguada, and Sabancuy. Although they are in a natural reserve, these nesting sites are threatened by agricultural activities and oil extraction. This study aimed to determine the presence and concentration of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in plasma and eggs and to assess the antioxidant response in plasma of nesting C. mydas from the southern Gulf of Mexico. Using censored statistics allowed us to do a realistic calculation incorporating the presence of non-detects in the analysis. A few contaminants (α+β+γ-HCH, trans-chlordane, 4,4′-DDE, 4,4′-DDT, methoxychlor, naphthalene, acenaphthylene, 2-Bromo naphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, and dibenz[a,h]anthracene) were detected in plasma and their corresponding eggs, but correlations were not-significant. Spatial differences in concentrations of persistent organic pollutants (POPs) among nesting sites may reflect differences in foraging areas. Approximately 30% of the POPs in plasma and 60% of the POPs in eggs correlated with the biomarkers of oxidative stress. Detection of POPs in C. mydas indicated that plasma and eggs are suitable matrices to assess POPs concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large numbers of organic compounds have been released into the marine environment due to industrial, agricultural, and other anthropogenic activities (Dachs and Méjanelle 2010). As a result, persistent organic pollutants (POPs) have become a significant concern among these organic compounds because of their potential hazard to marine species (Jakimska et al. 2011). POPs, such as organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs), are recognized for their toxicity, persistence in the environment, resistance to degradation, and capability to bioaccumulate in tissues of living organisms (Devi 2020; Weltmeyer et al. 2021).
The southern Gulf of Mexico region hosts an IUCN Index site for the green turtle, Chelonia mydas (Seminoff 2004). Three of these nesting sites occur along the coast of Campeche, namely Carmen, Isla Aguada, and Sabancuy (Guzmán and García 2016). Nevertheless, the southern Gulf of Mexico accounts for 76% of Mexican oil and gas production (Schifter et al. 2005). Furthermore, elevated PAHs and OCPs have been detected in the marine environment and some species on the southern coast of Campeche (Ponce Vélez and Botello 2005). These POPs could have entered this marine environment through oil extraction and agriculture activities (Carvalho et al. 2009; Rosano-Hernandez et al. 2012).
Green turtles are exposed to POPs via sediments, water, and food and can accumulate these pollutants in their tissues (Jakimska et al. 2011; Gallen et al. 2019). However, there are still gaps in this species’ status of POPs exposure. Adverse effects of POPs have been suggested on sea turtles (Finlayson et al. 2016). During vitellogenesis, lipids and pollutants move from the maternal bloodstream to the developing eggs (Miller and Limpus 2003; Guirlet et al. 2010; van de Merwe et al. 2010). Reactive oxygen species (ROS) are produced during normal cellular metabolism; however, oxidative stress occurs when there is an imbalance between the production of ROS and antioxidant defense (Valavanidis et al. 2006). As a defense mechanism against oxidative stress and damage, organisms have developed systems that involve enzymatic and non-enzymatic antioxidants (Valko et al. 2007). Evidence shows that exposure to POPs, including PAHs and OCPs, may induce oxidative stress, causing alterations in antioxidant defense and damage to lipids, proteins, and DNA (van der Oost et al. 2003). The antioxidant system can be altered by exposure to different stressors, including chemical pollutants; their quantification and the presence of cellular damage due to oxidative stress has been used as a biomarker in many studies (Bal et al. 2022; Morão et al. 2022).
Although green turtles are regarded as species of conservation concern and are considered sentinel organisms in marine biomonitoring (Aguirre and Lutz 2004), few studies have reported concentrations of POPs in green turtles from the Gulf of Mexico and the relationship between POPs exposure and indicators of oxidative stress (Swarthout et al. 2010; García-Besné et al. 2015; Salvarani et al. 2019). The present study measured the concentrations of 36 POPs in the plasma and eggs of green turtles nesting in three sites along the Campeche coast. In addition, it assessed the antioxidant response detected in the plasma of nesting C. mydas from the southern Gulf of Mexico as well as the variation of POP concentrations in female green turtles from three different nesting sites.
Materials and Methods
Study Site and Sample Collection
The fieldwork described in this study was conducted under Mexican regulations and permits provided by SEMARNAT (SGPA/DGVS/05845/13 and SGPA/DGVS/08106/14). From a conservation perspective, blood sampling is a non-invasive method of monitoring pollutants. On the other hand, using viable eggs is a destructive technique, and the number of eggs permitted is limited. Since one egg represents less than 1% of the total number of eggs that a female may lay in the entire season in the region, it is not possible to determine how POPs concentrations in the eggs vary.
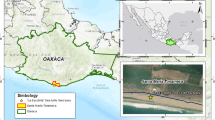
From July to September 2014, blood and eggs were collected from 29 female Chelonia mydas on 3 major nesting beaches along the Campeche coast in the southern Gulf of Mexico: Carmen 18°41′N, 91°42′W, Isla Aguada (IA) 18°47′N, 91°29′W, and Sabancuy (SB) 18°59′N, 91°11′W (Fig. 1). These beaches are located within the Laguna de Terminos Reserve. They are included in the IUCN Index site for green turtles in the southern Gulf of Mexico—an IUCN Index site is used to refer to beaches where nesting activity is intense, and long‐term nesting monitoring programs are developed to provide population trends (Seminoff 2004). However, agricultural activities in the surrounding areas may originate the release of organochlorine and organophosphorus pesticides into the marine ecosystem (Carvalho et al. 2009). Furthermore, oil and gas production has chronically impacted almost 300 km of the southern coast of the Gulf of Mexico (Rosano-Hernandez et al. 2012).
Nesting sites were surveyed at night to locate female green turtles in oviposition activity. One egg was randomly collected at oviposition or immediately after the egg-laying process, rinsed with deionized water, and wrapped in aluminum foil. Once the female laid eggs, blood collection was performed on each female, and two morphometric characters were measured: curved carapace length (CCL) and curved carapace width (CCW). Blood samples (6–10 mL) were collected from the dorsal cervical sinus (Owens and Ruiz 1980) using a disposable syringe and blood collection tubes containing sodium heparin. Blood samples were centrifuged at 2000 g for 10 min, and plasma was immediately separated. All samples were frozen in liquid nitrogen until analysis at the Laboratory of Environmental Coastal Sciences (Facultad de Química, Universidad Nacional Autónoma de México, Sisal, Yucatan, Mexico).
Sample Preparation and Chemical Analysis
Concentrations of pesticides (20 OCPs and Chlorpyrifos) and 16 PAHs in maternal plasma and eggs were determined by ultrasound-assisted extraction (USE) and solid-phase extraction (SPE) based on EPA methods 3550C, 3535, and 1699 (US EPA 2007a, b, c) and analytical procedures described in Trejo-Acevedo et al. (2009). For plasma analysis, 500 to 2000 μL were used, 2 mL of a saturated solution of ammonium sulfate (0.5 g/mL), and 2 mL of ethanol were added to achieve the precipitation and denaturation of proteins. USE was conducted twice with an ultrasonic processor (Cole Parmer model CPX500) at 70% amplitude for 2 min using 10 mL of hexane as an extraction solvent. The organic phase was separated by centrifugation (370 g for 8 min). Extracts were concentrated, and OCPs and PAHs were obtained by SPE using C18 500 mg/6 mL cartridges (Supelclean ENVI-18, 57064, Supelco). Cartridges were conditioned with 6 mL of dichloromethane, 6 mL of acetone, and 6 mL of hexane. Sample extracts were passed by gravity flow and finally eluted with 6 mL dichloromethane: hexane (3:7, v/v) by gravity flow and dried under vacuum for 1 min. The solvent retrieved was changed into ethyl acetate and concentrated using a gentle nitrogen flow, and samples were analyzed by GC–MS.
Egg content (yolk and albumen) was lyophilized for 72 h and then ground into powder. For each sample, 0.5 g of the lyophilized egg was extracted by USE with an ultrasonic processor (Cole Parmer model CPX500) at 70% amplitude for 2 min using 10 mL of hexane:acetone (1:1, v/v), and the organic phase was separated by centrifugation (1760 g for 10 min). Given the high concentration of lipids in turtle eggs, extracts were cleaned by dSPE using a kit roQ™ QhEChERS (KS0-8926, Phenomenex) to eliminate interferences caused by organic and acid compounds, pigments, and sugars. Cleaned extracts were concentrated, and OCPs and PAHs were obtained by SPE using Strata® Florisil 500 mg/6 mL cartridges (8B-S013-HCH, Phenomenex). First, cartridges were conditioned with 10 mL of hexane; then, sample extracts were passed by gravity flow. Finally, cartridges were eluted with 15 mL hexane:acetone (9:1, v/v) by gravity flow and dried under vacuum for 1 min. The solvent retrieved was changed into ethyl acetate, concentrated using a gentle nitrogen flow, and analyzed by GC–MS using deuterated PAHs as internal standards.
Plasma and egg extracts were analyzed on a gas chromatograph (Agilent Technologies, GC 6850 Series II) coupled to a mass selective detector (Agilent Technologies, 5975B VL MSD) with an automatic liquid sampler (Agilent Technologies, 7683B ALS). The injection was carried out in split-less mode at 280 °C. Chromatographic separation was performed by silica capillary column Zebron ZB-5MSi (Phenomenex). The carrier gas was He (ultra-pure grade) with a flow rate of 0.8 mL/min. Each sample was analyzed using two different chromatographic conditions. For OCPs, the oven temperature was initially set at 50 °C, increased 10 °C/min to 180 °C, then raised 1.5 °C/min to 200 °C (hold time 2 min), and finally increased 6 °C/min to 290 °C (hold time 1 min). For PAHs, the oven temperature was initially set at 60 °C, then increased 6 °C/min to 290 °C (hold time 11.67 min). Mass spectra (m/z 50–550) were recorded at a rate of five scans per second with an energy of 70 eV. Mass spectrometric analysis for quantitative measurement of the analytes was performed by electronic impact (EI) in selected ion monitoring (SIM) mode by use of two characteristic fragment ions for each chemical, one target and one qualifier ion (Supplementary Tables 1 and 2). Analytical quality control included procedural blanks, calibration curves using analytical standards (Chlorpyrifos PESTANAL® 45395-100MG, Pesticide 8081 Std Mix Supelco CRM46845, QTM PAH Mix Supelco CRM47930), and internal standards.
Antioxidant Activity
Oxidative stress biomarkers measured in this study included three antioxidant enzymes (SOD, GPx, and GST), total GSH, and lipid hydroperoxides. SOD was evaluated using a Sigma-Aldrich assay kit (19160), the reaction of which produces a water-soluble formazan dye upon the reduction of radical superoxide. The reduction rate of the superoxide radical is linearly related to the xanthine oxidase activity of the sample and is inhibited by SOD. GPx activity was determined indirectly by the oxidation of GSH to oxidized glutathione (GSSG) catalyzed by GPx (Paglia and Valentine 1967). GST activity was measured by the conjugation of reduced glutathione (GSH) and 1-chloro-2,4-dinitrobenzene, leading to the formation of glutathione-dinitrobenzene conjugate at 340 nm (Habig et al. 1974). Total GSH was measured using the Sigma-Aldrich Glutathione assay kit (CS0260). This kit uses an enzymatic recycling method with glutathione reductase (Baker et al. 1990); the GSH sulfhydryl group reacts with Ellman’s reagent and produces a yellow-colored compound read at 405 nm. Lipid hydroperoxides (LH) were measured using a modified ferrous oxidation-xylenol orange (FOX) method using the PeroxiDetect Kit (PD1, Sigma-Aldrich) following the manufacturer’s instructions (Rodríguez-Fuentes et al. 2017). The procedure is based on iron oxidation by peroxide that forms a coloring component with xylenol orange at acidic pH measured at 560 nm.
Data Analysis
Given the high number of non-detected POPs concentrations in egg and plasma samples, data were analyzed using statistics for censored data (Helsel 2012). Data were arranged in a censored matrix to compute summary statistics and totals. For each pollutant, an extra column indicates whether the included value was censored (below the method detection limit). A robust regression on order (RROS) method using the cenros function in NADA for R (Helsel 2012) was used to estimate all sites and per site mean, median, and standard deviation (SD). ANOVA was used to evaluate differences in morphometric variables between sites.
For multivariate analysis of pollutants, permutational MANOVA (PERMANOVA) and principal coordinate analysis (PCO) were performed, including as variables pollutants that presented less than 80% of censored data (below the method detection limit, Helsel 2012) using Primer v 7.0 + PERMANOVA add on. Data was set as an ordinal rank matrix, and then resemblance was calculated using each set’s Euclidean distance of samples (Legendre and Legendre 1998). Finally, permutational multiple paired t tests were applied to compare the centroids of the different sites in each data set; 9999 unrestricted permutations were used to generate the empirical F and t distributions (Anderson 2001).
For multivariate analysis of biomarkers, PERMANOVA and PCO were used. Data were transformed using the function log10(x+1) and normalized. The resemblance was calculated using the Euclidean distance of samples (Legendre and Legendre 1998). As described above, permutational multiple paired t tests were used to compare the centroids of the different sites in each data set.
Censored regressions were done using the cenreg command in NADA for R between a censored Y value (pollutant concentration) and an uncensored X variable (biomarker). The site was included in the regression as a factor. For correlation between data that had uncensored values, Spearman rank-order correlation was used using Statistica v 7.0.
Results
The mean values of CCL and CCW of nesting female C. mydas from the southern Gulf of Mexico are shown in Table 1. The size of females did not show statistically significant differences between nesting sites.
Pesticides detected in the plasma of female C. mydas nesting in three sites along the Campeche coast in the southern Gulf of Mexico are presented in Supplementary Table 3; 11 of 21 analyzed pesticides in plasma showed less than 80% of censored data. Endosulfan sulfate and cis-Chlordane were not detected in any plasma sample. PERMANOVA indicated significant differences between sites (Supplementary Table 4), demonstrating differences in the composition of the prevalent pesticides. OCPs content in turtles from Carmen was significantly different from turtles from Isla Aguada and Sabancuy (Supplementary Table 5). PCO analysis explained 50.5% of the total variation in the first two axes; PCO1 was strongly associated with heptachlor concentrations (Fig. 2).
The analysis of eggs indicated that 12 of 21 analyzed pesticides showed less than 80% of censored values (Supplementary Table 6). Endosulfan sulfate and cis-Chlordane were not detected in any egg. PERMANOVA indicated significant differences between sites (Supplementary Table 4). Figure 3 shows the result of the PCO that explained 65% of the total variation in the first two axes. Endrin, α+β+γ-HCH, and Aldrin were the prevalent pesticides in Isla Aguada and Carmen turtles. The rest of the pesticides are more associated with turtles sampled at Sabancuy. Permutational multiple paired t tests indicated statistical differences between the three sites (Supplementary Table 5).
Spearman correlation in OCs present in plasma and eggs with less than 80% censored data; α+β+γ-HCH, trans-chlordane, 4,4′-DDE, 4,4′-DDT, and methoxychlor were the pesticides with these characteristics. No significant correlations were found with the concentration found in plasma and eggs. Some OCPs (δ-HCH, heptachlor, and endrin ketone) were detected with less than 80% censored values in plasma but not in eggs. In contrast, 4,4′-DDD, aldrin, dieldrin, endrin, and endrin aldehyde were detected with less than 80% of censored values in eggs but not in plasma (Supplementary Tables 3 and 6).
Concerning PAHs concentrations in plasma, all 15 tested compounds were present with less than 80% of censored values (Supplementary Table 7). PERMANOVA indicated significant differences between sites demonstrating differences in the composition of the predominant PAHs in plasma (Supplementary Table 4). In addition, permutational multiple paired t tests indicated significant differences between the three sampling sites (Supplementary Table 5). PCO analysis was able to explain in the first two axes 38.6% of the total variation (Fig. 4).
PAH concentrations in eggs showed that 10 of 15 tested compounds were present with less than 80% of censored values (Supplementary Table 8). PERMANOVA indicated significant differences between sites (Supplementary Table 4). Permutational multiple paired t tests indicated significant differences between the three sampling sites (Supplementary Table 6); PCO explained 48.9% of the total variation in the first two axes (Fig. 5).
Naphthalene, acenaphthylene, 2-Bromo naphthalene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, and dibenz[a,h]anthracene were present in both eggs and plasma with less than 80% censored data. Spearman correlations between the concentration of these PAHs in plasma and eggs indicated that they were not statistically significant. Benz[a]anthracene + chrysene, benzo[b]fluoranthene, benzo(a)pyrene, indeno[1,2,3-cd]pyrene, and benzo[ghi]perylene were present in plasma with less than 80% of censored values, but not in the eggs (Supplementary Tables 7 and 8).
The results of the measured biomarkers are presented in Table 2. PERMANOVA indicated significant differences between nesting sites (Supplementary Table 3), with Carmen as the site that was different from the other sites (Supplementary Table 4). PCO analysis explained 69% of the total variation in the first two axes. PCO1 was associated with GPx activities and total GSH concentration; PCO2 was associated with SOD and GST activities (Fig. 6).
Results of the significant likelihood-r correlation between biomarkers, pollutants, and morphometric variables are presented in Tables 3 and 4 for plasma and egg, respectively. The correlations between morphometric variables and biomarkers were not significant.
Discussion
Few studies report the concentrations of OCPs in the plasma of C. mydas worldwide. In the present study, endrin ketone and α+β+γ-HCH were the OCPs detected at the highest level in plasma. García-Besné et al. (2015) analyzed OCPs in the blood of 32 nesting C. mydas sampled in Sabancuy and Punta Xen, Campeche; mirex, endrin ketone, γ-HCH, 4,4′-DDT, and 2,4-DDE were detected in more samples (34 to 53% of the turtles) and mean levels of these compounds ranged from 0.9 to 8.6 ng/g lipid fraction. Reports of OCPs in blood or plasma worldwide indicate the presence of 4,4′-DDE, 4,4′-DDT, dieldrin, α-HCH, β-HCH, γ-HCH, heptachlor, and trans-chlordane at the highest concentrations (Swarthout et al. 2010; van de Merwe et al. 2010; Komoroske et al. 2011; Labrada-Martagón et al. 2011; Camacho et al. 2014). Some OCPs are restricted or banned in many countries, Mexico included, because of their harmful environmental effects.
Results in eggs indicated that endrin and endrin aldehyde were the OCPs detected at the highest concentrations. García-Besné et al. (2015) reported 1250 ng/g lip of endrin ketone in one sample of C. mydas eggs collected in Campeche. On the other hand, Salvarani et al. (2019) mention that the sum of Drins (∑Drins) and iciathe sum of HCHs (∑HCHs) were the OCPs with the highest mean levels in C. mydas eggs collected in 2014 and 2015 in Isla Aguada and Punta Xen, Campeche (0.51 and 2.64 ng/g dw of ∑Drins and 0.49 and 4.93 ng/g dw of ∑HCHs, respectively).
To date, few studies have been performed to assess PAHs concentrations in the blood of sea turtles worldwide; these studies have been conducted on C. mydas, loggerhead, Caretta caretta, hawksbill turtles, Eretmochelys imbricata (Camacho et al. 2012, 2013, 2014; Bucchia et al. 2015; Casini et al. 2018; Vijayasarathy et al. 2019). In this study, indeno[1,2,3-cd]pyrene, benzo[a]pyrene, and benzo[b]fluoranthene were the PAHs with the highest concentration in the plasma of C. mydas. Indene[1,2,3-cd]pyrene is primarily found in gasoline and diesel exhaust, coal tar, and petroleum asphalt. Benzo[a]pyrene and benzo[b]fluoranthene are mainly found in gas and diesel exhaust, coal tar, amino acids, fatty acids, carbohydrate pyrolysis products, creosote oil, petroleum asphalt, and shale oils.
Reports of PAHs worldwide present different distributions of the individual compounds found at the highest concentration; phenanthrene and low molecular weight PAHs were detected at the highest levels in plasma and total blood of sea turtles C. caretta sampled in the Canary Islands and Cape Verde (Camacho et al. 2012) and Mediterranean Sea (Casini et al. 2018), respectively.
POPs found in the present study have been reported in water, sediment, and biota collected on the coast of Campeche and the Gulf of Mexico (Vázquez-Botello et al. 1993; Noreña-Barroso et al. 1999; Díaz-González et al. 2005; Ponce Vélez and Botello 2005; Rendón von Osten et al. 2005; Carvalho et al. 2009; Quetz et al. 2009).
Although these nesting sites are located within the protected Laguna de Terminos Reserve, they are influenced by several sources of pollution. Laguna de Terminos is the second-largest estuarine system in the Gulf of Mexico, receiving discharges from four main rivers (Usumacinta, Palizada, Chumpan, and Candelaria). Agricultural activities are also carried out in the surrounding areas, releasing pesticides into the estuarine system. Moreover, oil extraction facilities are located 100 km from the nesting sites, resulting in intense oil-related activities between offshore platforms and the study area. OCPs enter via runoff from agricultural areas where these pesticides were used; sources of PAHs may include oil spills and oil-related activities (transportation, storage, and use of crude oil) and combustion of fossil fuels and biomass. Although comparisons between reported POPs concentrations are complicated by the numerous factors that may differ between studies, the composition and concentration of POPs in C. mydas in this study may reflect differences in the contaminant profiles of their foraging grounds since pollutants are ingested primarily through food (Finlayson et al. 2016) and green turtles exhibit high levels of fidelity to foraging areas (Broderick et al. 2007). Differences in the present study concerning the nesting site may be related to the specific influence of the sources of pollutants, for example, proximity to the rivers, oil-related activities, or the urban nucleus. The currents and other oceanographic variables may also affect the transport and distribution of pollutants.
Some studies on sea turtles prove that POPs are transferred from maternal blood to eggs (Guirlet et al. 2010; van de Merwe et al. 2010; Stewart et al. 2011). In this study, few OCPs and PAHs detected in the plasma of nesting females were found in their corresponding egg, and no statistically significant relationship was found. The lack of a significant correlation between POP concentrations in maternal plasma and those in egg samples in this study could be explained because POPs in female plasma reflect recent exposure to contaminated sources. In contrast, the concentrations of POPs in their corresponding egg comprise POPs that have already been mobilized and transferred during vitellogenesis several months earlier. There have been other studies on turtles where no significant correlations were found between POP concentrations in female plasma and eggs (Rauschenberger et al. 2004; García-Besné et al. 2015), like the findings in the present work. Low molecular weight PAHs in eggs were more abundant and frequent than high molecular weight PAHs. Very few compounds of 5 or 6 rings, except for Dibenz[a,h]anthracene, were detected in the eggs. The abundance and frequency of low molecular weight PAHs in eggs may suggest preferential transfer of less lipophilic POPs from the maternal blood to eggs. Selective removal of less lipophilic POPs from the organism to eggs has been previously reported in sea turtles (Mckenzie et al. 1999; Guirlet et al. 2010; Stewart et al. 2011). Differences in the speed of POPs diffusion from the bloodstream to the different tissues may explain the lower content of highly lipophilic compounds (higher Kow values) in eggs compared to plasma levels (Muñoz and Vermeiren 2020).
Several studies involving sea turtles have assessed growth-related variations in POPs concentrations using carapace lengths (Mckenzie et al. 1999; Komoroske et al. 2011; Camacho et al. 2014) since no reliable age determination methods exist for sea turtles (Bjorndal et al. 1998). The present study used censored regression using the maximum likelihood correlation coefficient to evaluate the correlation between the concentration of POPs found in plasma and eggs and the morphometric data. Like the findings in the present work (where approximately 30% of the pollutants in plasma and 60% of contaminants in egg presented a negative correlation with female morphometry), some authors have reported negative relationships between body size and POPs concentrations in green turtles from other regions (Mckenzie et al. 1999; Richardson et al. 2010; Malarvannan et al. 2011).
A shift in feeding ecology might explain the negative correlations. For example, green turtles change from omnivorous as juveniles to herbivorous diets during adulthood (Bjorndal 1997). This change reduces the ingestion of POPs as the individual grows (Mckenzie et al. 1999; Keller 2013).
Despite the conservation status of C. mydas, there needs to be more information regarding the oxidative stress response against exposure to pollutants in this species. Few studies have been conducted on the antioxidant response of C. mydas performed in Baja, California, Mexico (Valdivia et al. 2007; Richardson et al. 2010; Labrada-Martagón et al. 2011). One more study reported oxidative stress indicators conducted on the hawksbill turtle, E. imbricata, nesting in Punta Xen, Campeche (Tremblay et al. 2017). In the present study, there was no relationship between morphometric variables and antioxidant response or oxidative damage in the plasma of nesting C. mydas. Similar results had been reported for C. mydas (Labrada-Martagón et al. 2011) and E. imbricata (Tremblay et al. 2017) and may be related to the similar size of the sampled organisms. Exposure to OCPs and PAHs may induce the production of ROS, leading to oxidative stress. The oxidative stress and excess ROS are counteracted by antioxidative defense mechanisms, which include the enzymes GPx, GST, and SOD, and the non-enzymatic antioxidants, i.e., GSH, vitamin C, and vitamin E, among others. GPx, SOD, GST, and lipid peroxidation levels have been proposed as valuable biomarkers for oxidative stress in aquatic organisms (Valavanidis et al. 2006). Multivariate analysis of POPs demonstrated differences in plasma and egg concentration in the different nesting sites that were studied in Campeche, Mexico; the regional separation of the sea turtles by their biomarkers had been previously related to differences in the habitat and environmental conditions (Labrada-Martagón et al. 2011).
In this study, GSH levels detected in the plasma of nesting C. mydas were negatively correlated with 29% of the pollutants contaminants in the plasma and 60% in the egg. GSH concentrations were strongly correlated with trans-Chlordane, endrin ketone, and Indeno 1,2,3-cd pyrene in plasma and to DDT and its metabolites, methoxychlor, anthracene, and fluoranthene in eggs. These results suggest a decrease in the total GSH level in the plasma of C. mydas exposed to pollutants. The reduction in GSH concentration may be attributed to the utilization of this compound as an antioxidant and an essential conjugant in the phase II metabolism of these pollutants. The decrease in GSH levels due to exposure to contaminants has also been reported in other aquatic species (Sanchez et al. 2007; Karadag et al. 2014). GPx activity in the plasma of nesting C. mydas was negatively correlated to 33% of the pollutants present in plasma and 70% of the contaminants present in the egg; it was strongly correlated to concentrations of 4,4′ DDE and endrin ketone in plasma, and DDT and its metabolites, methoxychlor, anthracene, and fluoranthene in eggs. This result would indicate that the presence of these pollutants reduces GPx activity detected in plasma C. mydas. On the contrary, in C. mydas from Baja California, GPx activity was positively correlated with trans-chlordane (Labrada-Martagón et al. 2011). Still, this activity was monitored in erythrocytes, not plasma, an extracellular source of antioxidant enzymes.
SOD activity in the plasma of nesting C. mydas was negatively correlated to the concentration of 29% of the pollutants in the plasma and 65% of the contaminants in the egg. It was strongly correlated with 4-4′ DDE, endrin ketone in plasma, DDT metabolites, methoxychlor, anthracene, and fluoranthene in plasma. In accordance, Kovacik et al. (2019) negatively correlated the presence of contaminants with SOD and lipid peroxides in fish. This result would suggest reducing SOD activity in C. mydas exposed to these pollutants. In other studies, no relationship was detected between SOD activity and the concentration of contaminants in C. mydas from Baja California (Labrada-Martagón et al. 2011) and in E. imbricata from Campeche (Tremblay et al. 2017). However, other studies on fish species have shown an increase in SOD activity in organisms exposed to pollutants (Monteiro et al. 2010; Carvalho et al. 2012).
GST activities were negatively correlated to the concentration of 45% of the pollutants in the plasma and 60% in the egg. It was strongly related to 4-4′DDE, endrin ketone in plasma, and DDT and its metabolites, aldrin, methoxychlor, anthracene, and fluoranthene in eggs. In previous reports in C. mydas from Baja California, GST activity was positively correlated to the concentrations of α-, β-, γ-HCH, ƩHCHs, heptachlor, and ƩHeptachlors (Labrada-Martagón et al. 2011).
In the plasma of nesting C. mydas, a negative correlation was detected between lipid peroxidation and 25% of the pollutants contaminants in the plasma and 60% in the egg. Exposure to pollutants, including OCPs and PAHs, may cause an increase in lipid peroxidation in fish species, as has been previously reported elsewhere (Avci et al. 2005; Otitoloju and Olagoke 2011). The results of biomarkers of oxidative stress are difficult to interpret and compare since there are tissue, concentration, and time dependency. It is not rare that for the same biomarker, some reports present an increase, others a no change, and even others have a reduction in enzyme activities or the concentration of antioxidants or oxidative damage indicators. As previously reported (van der Oost et al. 2003 and references therein), oxidative stress biomarkers are unspecific; they may or may not be related to the presence of pollutants since not all the contaminants (or their metabolites) produce ROS or oxidative stress. In addition, studies are showing an abnormal increase in ROS content under the activation of POPs in wildlife species (Tian et al. 2021, González-Mille et al. 2019). Oxidative stress biomarkers are also related to respiration, and any stressor may increase oxidative damage (for example, changes in oxygen concentration or temperature). It has been previously reported that other hypoxia-tolerant reptiles must deal with a significant amount of ROS generated due to ischemia-reperfusion associated with diving (Valdivia et al. 2007). Therefore, the measurement of oxidative stress is a valuable tool for the health assessment of endangered species, but these biomarkers should be interpreted carefully. The use of multivariate methods to assess the antioxidant status of organisms exposed to pollutants may be a valuable tool to understand what is happening in the sampled organism. For example, in the present study, PCO shows that the PCO1 axis is related to GPx and GSH and the presence of LH; PCO2 is related to Sand but also LH. It has been reported that SOD and CAT (the latter was not measured in the present study) are the first lines of defense against ROS, inhibiting superoxide produced during the metabolism of some pollutants or cellular respiration (Dobal et al. 2022). On the other hand, GSH plays a double role as an antioxidant (working as an electron donor for GPx) and as a conjugant of phase II metabolism reactions catalyzed by GST. GST also has an essential role in the antioxidant system, reducing lipid hydroperoxides to alcohol (Regoli et al. 2014). LH acts as an indicator of oxidative stress. In the present study, turtles nesting in Sabancuy were associated with the concentration of LH; it is interesting to add that turtles nesting in this site also presented higher concentrations of some OCPs and PAHs that may be related to this finding. On the other hand, higher levels of SOD and GPx were present in turtles nesting in Carmen. This increase in activity may be a defense against oxidative damage with the consequent lower concentration of LH.
Conclusions
In the present study, concentrations of OCPs, PAHs, and chlorpyrifos were analyzed in the plasma and eggs of nesting female Chelonia mydas from the southern Gulf of Mexico, providing baseline data on POP concentrations in this species. The use of censored statistics allowed us to do a realistic calculation of summary statistics, multivariate procedures, and correlations incorporating non-detects in the analysis. Few compounds were detected in plasma and their corresponding eggs, and correlations were non-significant. Concentrations of POPs in the plasma of C. mydas showed a negative correlation with female morphometry. Spatial differences in concentrations of POPs among nesting sites may reflect differences in foraging areas. Detection of POPs in C. mydas indicated that plasma and eggs are suitable matrices to assess POP concentrations and multivariate analysis for biomarkers to determine the relationship between POPs and the antioxidant system and oxidative damage biomarkers.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguirre, A.A., and P. Lutz. 2004. Marine turtles as sentinels of ecosystem health: is fibropapillomatosis an indicator? EcoHealth 1: 275–283. https://doi.org/10.1007/s10393-004-0097-3.
Anderson, M.J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46. https://doi.org/10.1111/j.1442-9993.2001.tb00081.x.
Avci, A., M. Kacmaz, and I. Durak. 2005. Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicology and Environmental Safety 60: 101–105. https://doi.org/10.1016/j.ecoenv.2003.10.003.
Baker, M.A., G.J. Cerniglia, and A. Zaman. 1990. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Analytical Biochemistry 190: 360–365. https://doi.org/10.1016/0003-2697(90)90208-Q.
Bal, A., F. Panda, S.G. Pati, T.N. Anwar, K. Das, and B. Paital. 2022. Influence of anthropogenic activities on redox regulation and oxidative stress responses in different phyla of animals in coastal water via changing in salinity. Water 14 (24): 4026. https://doi.org/10.3390/w14244026.
Bjorndal, K.A. 1997. Foraging ecology and nutrition of sea turtles. In The biology of sea turtles, vol. 1, ed. P.L. Lutz and J.A. Musick, 199–231. Boca Raton: CRC Press. https://doi.org/10.1201/9780203737088.
Bjorndal, K.A., A.B. Bolten, R.A. Bennett, E.R. Jacobson, T.J. Wronski, J.J. Valeski, and P.J. Eliazar. 1998. Age and growth in sea turtles: limitations of skeletochronology for demographic studies. Copeia 1: 23–30. https://doi.org/10.2307/1447698.
Broderick, A.C., M.S. Coyne, W.J. Fuller, F. Glen, and B.J. Godley. 2007. Fidelity and over-wintering of sea turtles. Proceedings of the Biological Sciences 274: 1533–1539. https://doi.org/10.1098/rspb.2007.0211.
Bucchia, M., M. Camacho, M.R.D. Santos, L.D. Boada, P. Roncada, R. Mateo, M.E. Ortiz-Santaliestra, J. Rodríguez-Estival, M. Zumbado, J. Orós, L.A. Henríquez-Hernández, N. García-Álvarez, and O.P. Luzardo. 2015. Plasma levels of pollutants are much higher in loggerhead turtle populations from the Adriatic Sea than in those from open waters (Eastern Atlantic Ocean). Science of the Total Environment 523: 161–169. https://doi.org/10.1016/j.scitotenv.2015.03.047.
Camacho, M., L.D. Boada, J. Orós, P. Calabuig, M. Zumbado, and O.P. Luzardo. 2012. Comparative study of polycyclic aromatic hydrocarbons (PAHs) in plasma of Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Marine Pollution Bulletin 64: 1974–1980. https://doi.org/10.1016/j.marpolbul.2012.06.002.
Camacho, M., L.D. Boada, J. Orós, P. López, M. Zumbado, M. Almeida-González, and O.P. Luzardo. 2013. Comparative study of organohalogen contamination between two populations of Eastern Atlantic loggerhead sea turtles (Caretta caretta). Bulletin of Environment Contamination and Toxicology 91: 678–683. https://doi.org/10.1007/s00128-013-1123-3.
Camacho, M., L.D. Boada, J. Orós, P. López, M. Zumbado, M. Almeida-González, and O.P. Luzardo. 2014. Monitoring organic and inorganic pollutants in juvenile live sea turtles: results from a study of Chelonia mydas and Eretmochelys imbricata in Cape Verde. Science of the Total Environment 481: 303–310. https://doi.org/10.1016/j.scitotenv.2014.02.051.
Carvalho, F.P., J.P. Villeneuve, C. Cattini, J. Rendón, and J.M. De Oliveira. 2009. Ecological risk assessment of PCBs and other organic contaminant residues in Laguna de Terminos, Mexico. Ecotoxicology 18: 403–416. https://doi.org/10.1007/s10646-008-0295-9.
Carvalho, C.S., V.A. Bernusso, H.S.S. Araújo, E.L.G. Espíndola, and M.N. Fernandes. 2012. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 89: 60–69. https://doi.org/10.1016/j.chemosphere.2012.04.013.
Casini, S., I. Caliani, M. Giannetti, L. Marsili, S. Maltese, D. Coppola, N. Bianchi, T. Campani, S. Ancora, C. Caruso, G. Furii, M. Parga, A. D’Agostino, and M.C. Fossi. 2018. First ecotoxicological assessment of Caretta caretta (Linnaeus, 1758) in the Mediterranean Sea using an integrated nondestructive protocol. Science of the Total Environment 631–632: 1221–1233. https://doi.org/10.1016/j.scitotenv.2018.03.111. (PMID: 29727947).
Dachs, J., and L. Méjanelle. 2010. Organic pollutants in coastal waters, sediments, and biota: a relevant driver for ecosystems during the anthropocene? Estuaries and Coasts 33: 1–14. https://doi.org/10.1007/s12237-009-9255-8.
Devi, N.L. 2020. Persistent organic pollutants (POPs): environmental risks, toxicological effects, and bioremediation for environmental safety and challenges for future research. In Bioremediation of industrial waste for environmental safety, ed. G. Saxena and R. Bharagava, 458. Singapore: Springer. https://doi.org/10.1007/978-981-13-1891-7_4.
Díaz-González, G., A.V. Botello, and G. Ponce-Vélez. 2005. Plaguicidas organoclorados en pastos y peces de los sistemas Candelaria-Panlau y Palizada del Este, Laguna de Términos, Campeche, México. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, ed. A.V. Botello, J. Rendón-von Osten, G. Gold-Bouchot, and C. Agraz-Hernández, 207–223. México: Univ Autón de Campeche, Univ Nal Autón de México, Instituto Nacional de Ecología.
Dobal, V., P. Suárez, Y. Ruiz, O. García-Martín, and F. San Juan. 2022. Activity of antioxidant enzymes in Mytilus galloprovincialis exposed to tar: integrated response of different organs as pollution biomarker in aquaculture areas. Aquaculture 548: 737638. https://doi.org/10.1016/j.aquaculture.2021.737638.
Finlayson, K.A., F.D.L. Leusch, and J.P. van de Merwe. 2016. The current state and future directions of marine turtle toxicology research. Environment International 94: 113–123.
Gallen, C., A.L. Heffernan, S. Kaserzon, G. Dogruer, S. Samanipour, M.J. Gomez-Ramos, and J.F. Mueller. 2019. Integrated chemical exposure assessment of coastal green turtle foraging grounds on the Great Barrier Reef. STOTEN 657: 401–409. https://doi.org/10.1016/j.scitotenv.2018.11.322.
García-Besné, G., C. Valdespino, and J. Rendón-von Osten. 2015. Comparison of organochlorine pesticides and PCB residues among hawksbill (Eretmochelys imbricata) and green (Chelonia mydas) turtles in the Yucatan Peninsula and their maternal transfer. Marine Pollution Bulletin 91: 139–148. https://doi.org/10.1016/j.marpolbul.2014.12.015.
González-Mille, D.J., C.A. Ilizaliturri-Hernández, G. Espinosa-Reyes, O. Cruz-Santiago, M.D.C. Cuevas-Díaz, C.C. Martín Del Campo, and R. Flores-Ramírez. 2019. DNA damage in different wildlife species exposed to persistent organic pollutants (POPs) from the delta of the Coatzacoalcos river, Mexico. Ecotoxicology and Environmental Safety 180: 402–411. https://doi.org/10.1016/j.ecoenv.2019.05.030.
Guirlet, E., K. Das, J.P. Thome, and M. Girondot. 2010. Maternal transfer of chlorinated contaminants in the leatherback turtles, Dermochelys coriacea, nesting in French Guiana. Chemosphere 79: 720–726. https://doi.org/10.1016/j.chemosphere.2010.02.047.
Guzmán, V., and P.A. García. 2016. Informe Técnico 2015 del programa de Conservación de Tortugas Marinas en Laguna de Términos, Campeche, México. Campeche: APFFLT/RPCyGM/CONANP.
Habig, W.H., M.J. Pabst, and W.B. Jakoby. 1974. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry 249: 7130–7139.
Helsel, D.R. 2012. Statistics for censored environmental data using Minitab and R, 344. Hoboken, New Jersey: John Wiley & Sons, Inc. https://doi.org/10.1002/9781118162729.
Jakimska, A., P. Konieczka, K. Skóra, and J. Namieśnik. 2011. Bioaccumulation of metals in tissues of marine animals, part II: metal concentrations in animal tissues. Polish Journal of Environmental Studies 20: 1127–1146.
Karadag, H., Ö. Firat, and Ö. Firat. 2014. Use of oxidative stress biomarkers in Cyprinus carpio L. for the evaluation of water pollution in ataturk dam lake (Adiyaman, Turkey). Bulletin of Environment Contamination and Toxicology 92: 289–293. https://doi.org/10.1007/s00128-013-1187-0.
Keller, J.M. 2013. Exposure to and effects of persistent organic pollutants. In The biology of sea turtles, vol. III, ed. J. Wyneken, K.J. Lohmann, and J.A. Musick, 285–328. Boca Raton: CRC Press.
Komoroske, L.M., R.L. Lewison, J.A. Seminoff, D.D. Deheyn, and P.H. Dutton. 2011. Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere 84: 544–552. https://doi.org/10.1016/j.chemosphere.2011.04.023.
Kovacik, A., E. Tvrda, M. Miskeje, et al. 2019. Trace metals in the freshwater fish Cyprinus carpio: effect to serum biochemistry and oxidative status markers. Biological Trace Element Research 188: 494–507. https://doi.org/10.1007/s12011-018-1415-x.
Labrada-Martagón, V., P.A. Tenorio Rodríguez, L.C. Méndez-Rodríguez, and T. Zenteno-Savín. 2011. Oxidative stress indicators and chemical contaminants in East Pacific green turtles (Chelonia mydas) inhabiting two foraging coastal lagoons in the Baja California Peninsula. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 154: 65–75. https://doi.org/10.1016/j.cbpc.2011.02.006.
Legendre, P., and L. Legendre. 1998. Numerical ecology, vol. 24, 852. Amsterdam: Elsevier.
Malarvannan, G., S. Takahashi, T. Isobe, T. Kunisue, A. Sudaryanto, T. Miyagi, M. Nakamura, S. Yasumura, and S. Tanabe. 2011. Levels and distribution of polybrominated diphenyl ethers and organochlorine compounds in sea turtles from Japan. Marine Pollution Bulletin 63: 172–178. https://doi.org/10.1016/j.marpolbul.2011.02.010.
McKenzie, C., B.J. Godley, R.W. Furness, and D.E. Wells. 1999. Concentrations and patterns of organochlorine contaminants in marine turtles from Mediterranean and Atlantic waters. Marine Environment Research 47: 117–135. https://doi.org/10.1016/S0141-1136(98)00109-3.
Miller, J.D., and C.J. Limpus. 2003. Ontogeny of marine turtle gonads. In The biology of sea turtles, vol. 2, ed. P.L. Lutz, J.A. Musick, and J. Wyneken, 199–224. Washington DC: CRC Press. https://doi.org/10.1201/9781420040807.ch7.
Monteiro, D.A., F.T. Rantin, and A.L. Kalinin. 2010. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19: 105–123. https://doi.org/10.1007/s10646-009-0395-1.
Morão, I.F.C., M.F.L. Lemos, R. Félix, S. Vieira, C. Barata, and S.C. Novais. 2022. Stress response markers in the blood of São Tomé green sea turtles (Chelonia mydas) and their relation with accumulated metal levels. Environmental Pollution 293: 118490. https://doi.org/10.1016/j.envpol.2021.118490.
Muñoz, C.C., and P. Vermeiren. 2020. Maternal transfer of persistent organic pollutants to sea turtle eggs: a meta-analysis addressing knowledge and data gaps toward an improved synthesis of research outputs. Environmental Toxicology and Chemistry 39: 9–29. https://doi.org/10.1002/etc.4585.
Noreña-Barroso, E., G. Gold-Bouchot, O. Zapata-Perez, and J.L. Sericano. 1999. Polynuclear aromatic hydrocarbons in American oysters Crassostrea virginica from the Terminos Lagoon, Campeche, Mexico. Marine Pollution Bulletin 38: 637–645. https://doi.org/10.1016/S0025-326X(98)00165-9.
Otitoloju, A., and O. Olagoke. 2011. Lipid peroxidation and antioxidant defense enzymes in Clarias gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons. Environmental Monitoring and Assessment 182: 205–213. https://doi.org/10.1007/s10661-010-1870-0.
Owens, D.W., and G.J. Ruiz. 1980. New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36: 17–20.
Paglia, D.E., and W.N. Valentine. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine 70: 158–169.
Ponce Vélez, G., and A.V. Botello. 2005. Niveles de hidrocarburos en el Golfo de México. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, ed. A.V. Botello, J. Rendón-von Osten, G. Gold-Bouchot, and C. Agraz-Hernández, 269–2998. México: Univ Autón de Campeche, Univ Nal Autón de México, Instituto Nacional de Ecología.
Quetz, L., M. Memije, J. Benítez, and J. Rendón-von Osten. 2009. Hidrocarburos aromáticos policíclicos en sedimentos del río y costa de Champotón, Campeche. Jaina 20: 27–34.
Rauschenberger, R.H., M.S. Sepúlveda, J.J. Wiebe, N.J. Szabo, and T.S. Gross. 2004. Predicting maternal body burdens of organochlorine pesticides from eggs and evidence of maternal transfer in Alligator mississippiensis. Environmental Toxicology and Chemistry 23: 2906–2915. https://doi.org/10.1897/03-584.1.
Regoli, F., D. Pellegrini, A.M. Cicero, et al. 2014. A multidisciplinary weight of evidence approach for environmental risk assessment at the Costa Concordia wreck: integrative indices from Mussel Watch. Marine Environment Research 96: 92–104. https://doi.org/10.1016/j.marenvres.2013.09.016.
Rendón von Osten, J., M. Memije Canepa, and N.A. Ek Moo. 2005. Plaguicidas orgánicos persistentes (POPs) en sedimentos de la costa sur de Campeche, México. In Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, ed. A.V. Botello, J. Rendón-von Osten, G. Gold-Bouchot, and C. Agraz-Hernández, 249–260. México: Univ Autón de Campeche, Univ Nal Autón de México, Instituto Nacional de Ecología.
Richardson, K.L., M. Lopez Castro, S.C. Gardner, and D. Schlenk. 2010. Polychlorinated biphenyls and biotransformation enzymes in three species of sea turtles from the Baja California peninsula of Mexico. Archives of Environmental Contamination and Toxicology 58: 183–193. https://doi.org/10.1007/s00244-009-9360-5.
Rodríguez-Fuentes, G., M. Murúa-Castillo, F. Díaz, C. Rosas, C. Caamal-Monsreal, A. Sánchez, K. Paschke, and C. Pascual. 2017. Ecophysiological biomarkers defining the thermal biology of the Caribbean lobster Panulirus argus. Ecological Indicators 78: 192–204. https://doi.org/10.1016/j.ecolind.2017.03.011.
Rosano-Hernandez, M.C., H. Ramirez-Saad, and L. Fernandez-Linares. 2012. Petroleum-influenced beach sediments of the Campeche Bank, Mexico: diversity and bacterial community structure assessment. Journal of Environmental Management 95: S325–S331. https://doi.org/10.1016/j.jenvman.2011.06.046.
Salvarani, P.I., F. Morgado, L.R. Vieira, and J.R.V. Osten. 2019. Organochlorines contaminants in eggs of hawksbill (Eretmochelys imbricata) and green sea turtles (Chelonia mydas) from Mexico coast. Archives of Environmental Contamination and Toxicology 76 (3): 425–434.
Sanchez, W., S. Aït-Aïssa, O. Palluel, J.M. Ditche, and J.M. Porcher. 2007. Preliminary investigation of multi-biomarker responses in three-spined stickleback (Gasterosteus aculeatus L.) sampled in contaminated streams. Ecotoxicology 16: 279–287. https://doi.org/10.1007/s10646-006-0131-z.
Schifter, I., C. González-Macías, A. Miranda, and E. López-Salinas. 2005. Air emissions assessment from offshore oil activities in Sonda de Campeche, Mexico. Environmental Monitoring and Assessment 109: 135–145. https://doi.org/10.1007/s10661-005-5844-6.
Seminoff, J.A. 2004. Chelonia mydas. The IUCN Red List of Threatened Species 2004: e.T4615A11037468. https://doi.org/10.2305/IUCN.UK.2004.RLTS.T4615A11037468.en.
Stewart, K.R., J.M. Keller, R. Templeton, J.R. Kucklick, and C. Johnson. 2011. Monitoring persistent organic pollutants in leatherback turtles (Dermochelys coriacea) confirms maternal transfer. Marine Pollution Bulletin 62: 1396–1409. https://doi.org/10.1016/j.marpolbul.2011.04.042.
Swarthout, R.F., J.M. Keller, M. Peden-Adams, A.M. Landry, P.A. Fair, and J.R. Kucklick. 2010. Organohalogen contaminants in blood of Kemp’s Kemp’s ridley (Lepidochelys kempii) and green sea turtles (Chelonia mydas) from the Gulf of Mexico. Chemosphere 78: 731–741. https://doi.org/10.1016/j.chemosphere.2009.10.059.
Tian, Y., L. Pan, J. Miao, F. Lei, R. Xu, and X. Zhang. 2021. The mechanism of apoptosis of Chlamys farreri hemocytes under benzopyrene stress in vitro. Science of the Total Environment 794: 148731. https://doi.org/10.1016/j.scitotenv.2021.148731.
Trejo-Acevedo, A., F. Díaz, L. Carrizales, et al. 2009. Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere 74: 975–976. https://doi.org/10.1016/j.chemosphere.2008.10.030.
Tremblay, N., A. Ortíz Arana, M. González Jáuregui, and J. Rendón-von Osten. 2017. Relationship between organochlorine pesticides and stress indicators in hawksbill sea turtle (Eretmochelys imbricata) nesting at Punta Xen (Campeche), Southern Gulf of Mexico. Ecotoxicology 26: 173–183. https://doi.org/10.1007/s10646-016-1752-5.
US EPA United States Environmental Protection Agency. 2007a. Method 3550C: ultrasonic extraction. https://www.epa.gov/sites/default/files/2015-12/documents/3550c.pdf. Accessed 17 Oct 2014.
US EPA United States Environmental Protection Agency. 2007b. Method 3535A: solid-phase extraction. Revision 1. https://www.epa.gov/sites/default/files/2015-12/documents/3535a.pdf. Accessed 17 Oct 2014.
US EPA United States Environmental Protection Agency. 2007c. Method 1699: pesticides in water, soil, sediment, biosolids, and tissue by HRGC/HRMS. https://www.epa.gov/sites/default/files/2015-10/documents/method_1699_2007.pdf. Accessed 17 Oct 2014.
Valavanidis, A., T. Vlahogianni, M. Dassenakis, and M. Scoullos. 2006. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety 64: 178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013.
Valdivia, P.A., T. Zenteno-Savín, S.C. Gardner, and A. Alonso Aguirre. 2007. Basic oxidative stress metabolites in eastern Pacific green turtles (Chelonia mydas agassizii). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 146: 111–117. https://doi.org/10.1016/j.cbpc.2006.06.008.
Valko, M., D. Leibfritz, J. Moncol, M.T.D. Cronin, M. Mazur, and J. Telser. 2007. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology 39: 44–84. https://doi.org/10.1016/j.biocel.2006.07.001.
van de Merwe, J.P., M. Hodge, J.M. Whittier, K. Ibrahim, and S.Y. Lee. 2010. Persistent organic pollutants in the green sea turtle Chelonia mydas: nesting population variation, maternal transfer, and effects on development. Marine Ecology Progress Series 403: 269–278. https://doi.org/10.3354/meps08462.
van der Oost, R., J. Beyer, and N.P.E. Vermeulen. 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology 13: 57–149. https://doi.org/10.1016/S1382-6689(02)00126-6.
Vázquez-Botello, A., G. Ponce-Vélez, and G. Díaz-González. 1993. Hidrocarburos aromáticos policíclicos (PAH’s) en áreas costeras del Golfo de México. Hidrobiológica 3: 1–15.
Vijayasarathy, S., C. Baduel, C. Hof, I. Bell, M. del Mar Gómez Ramos, M.J.G. Ramos, M. Kock, and C. Gaus. 2019. Multi-residue screening of non-polar hazardous chemicals in green turtle blood from different foraging regions of the Great Barrier Reef. Science of the Total Environment 652: 862–868. https://doi.org/10.1016/j.scitotenv.2018.10.094.
Weltmeyer, A., G. Dogruer, H. Hellert, J.D. Ouellet, K. Townsend, A. Covaci, and L. Weijs. 2021. Distribution and toxicity of persistent organic pollutants and methoxylated polybrominated diphenylethers in different tissues of green turtle Chelonia mydas. Environmental Pollution 277: 116795. https://doi.org/10.1016/j.envpol.2021.116795.
Acknowledgements
The authors are grateful to Turtle Station Managers, Biol. Vicente Guzmán Hernández and Ing. Alfonso Díaz Molina, for the support given during the sample collection, Félix Canul Cejas for his guidance and advice during the blood collection. We thank Andrés Cruz Quintana for his assistance collecting blood samples and Mario Canul Parra for his technical support in the lab work.
Funding
This study was supported by Universidad Nacional Autónoma de México PAPIIT-DGAPA grants IA200214 and IA202416 and the Universidad Autónoma del Carmen FCN/2DOP2018/01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Blood was sampled under permits provided by SEMARNAT (SGPA/DGVS/05845/13 and SGPA/DGVS/08106/14). Blood samples were only collected under standardized procedures. No nesting female was harmed or killed during blood collection. Gerardo Rivas-Hernández was responsible for the collection of all blood samples.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Communicated by Wen-Xiong Wang
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rivas-Hernández, G., Rodríguez-Fuentes, G., Noreña-Barroso, E. et al. Biomarkers of Oxidative Stress and Persistent Organic Pollutants in Plasma and Eggs of Chelonia mydas Nesting in the Southern Gulf of Mexico. Estuaries and Coasts (2023). https://doi.org/10.1007/s12237-023-01190-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12237-023-01190-1